The Effect of SnO2 Addition on Sintering Behaviors in a Titanium Oxide-Copper Oxide System
Article information
Abstract
The low-temperature sinterability of TiO2-CuO systems was investigated using a solid solution of SnO2. Sample powders were prepared through conventional ball milling of mixed raw powders. With the SnO2 content, the compositions of the samples were Ti1-xSnxO2-CuO(2 wt.%) in the range of x ≤ 0.08. Compared with the samples without SnO2 addition, the densification was enhanced when the samples were sintered at 900°C. The dominant mass transport mechanism seemed to be grain-boundary diffusion during heat treatment at 900°C, where active grain-boundary diffusion was responsible for the improved densification. The rapid grain growth featured by activated sintering was also obstructed with the addition of SnO2. This suggested that both CuO as an activator and SnO2 dopant synergistically reduced the sintering temperature of TiO2.
1. Introduction
At present, considerable research attention is being paid to multilayer components in microelectronic devices and microwave communication systems. To manufacture devices, there is a need for integration and interconnection technologies in between ingredients. In this regard, a low temperature cofired ceramic (LTCC) technology enabling the fabrication of three-dimensional ceramic modules with low dielectric loss and internal electrodes is required. According to this, TiO2 with a high dielectric constant (~100) and low dielectric loss has been widely investigated as an LTCC material [1-3].
However, pure TiO2 requires a high sintering temperature(> 1200°C) to obtain a high density. Therefore, in attempts to apply TiO2 to the LTCC material, it is necessary to reduce the sintering temperature to below the melting temperatures of internal conductors such as silver and copper [2]. In this context, CuO as an activator was added into TiO2 matrix. The addition of CuO has been proven to be an effective way to reduce the sintering temperature of TiO2. However, the TiO2-CuO system has a drawback of rapid grain growth during heat-treatment [4, 5]. It would therefore be meaningful to find an additive to deter rapid grain growth for the TiO2-CuO system. This is why hindering the rapid grain growth is beneficial for enhancing densification.
Pure SnO2 is also very difficult to densify [6, 7]. CuO is an effective additive to densify SnO2 [8, 9]. Recently, considerable attention has been paid to the application of solid solutions of TiO2-SnO2 systems in electronic devices such as gas sensors [10] or varistors [11, 12]. In accordance with this, SnO2 could be considered an effective additive to densify TiO2-CuO systems. Therefore, in the present study, densification and grain growth behavior were investigated with the variation of SnO2 content for the TiO2-CuO systems. As a result, the solid solution and activator at a low temperature of 900°C for Ti1-xSnxO2- CuO(2wt%) had a noticeable synergy effect of enhancing densification, but there was an optimum level of the addition of SnO2 to achieve a significant effect.
2. Experimental
Pure rutile TiO2 (Kojundo chemical Co., Japan; purity: 99.9%, rutile, 2 μm), CuO (Kojundo chemical Co., Japan; purity: 99.9%, 2 μm), and SnO2(Samchun pure chemical Co., South Korea; purity: 99.0%) were used as raw materials. Mixed powders of TiO2-SnO2-CuO(2wt%) were prepared through wet-milling for 24 h in a polyethylene bottle. Zirconia balls and an ethyl alcohol were used as the milling media and the solvent, respectively. Following ball milling, the powders were dried at 90°C for 24 h. The dried powders were then crushed and sieved (500 mesh). Next, ~2 g of each powder was uniaxially pressed into pellets under 0.5 MPa, after which the pellets were cold isostatically pressed under 100 MPa for 3 min. The green pellets were ~13 mm in diameter and 7 mm thick. The green compact pellets were sintered at 900°C under air in a MoSi2 resistance box furnace. The heating and cooling rates were ~5°C/min in both cases.
The heating proceeded at 900°C for up to 12 h. For the present samples, the amount of CuO was kept at 2 wt%, and the contents of SnO2 were varied up to 0.08 mol for the composition of Ti1-xSnxO2. The developed phases of the sintered body were analyzed using standard X-ray diffraction with a diffractometer (D/Max 2000, Rigaku Co., Japan). The sintered densities were obtained by the Archimedes method using distilled water as the immersion medium. Every density datum was determined based on an average of three similar specimens. Micrographs of the sintered samples were observed on the polished surfaces through scanning electron microscopy (VEGA II LMU, TESCAN). For the observation, the sample surfaces were polished with diamond pastes down to 1 μm. The polished samples were then thermally etched at 850°C for 30 min. The average grain sizes were determined from the SEM micrographs using the image analysis software package (VEGA II, TESCAN), while counting at least 300 grains for each sample.
3. Results and Discussion
3.1 The solution effect of SnO2 in TiO2-CuO(2wt%) sample
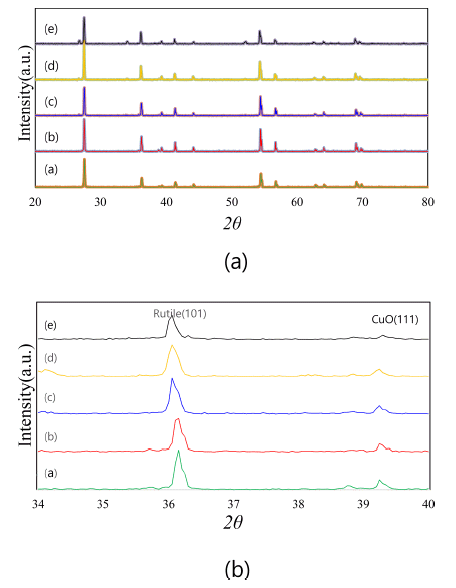
Figure 1 shows the X-ray diffraction patterns of (Ti,Sn) O2-CuO(2wt%) samples with varying SnO2 content. With variations in the SnO2 content, the compositions of the present samples are Ti1-xSnxO2-CuO(2 wt%) at x = 0, 0.01, 0.02, 0.04, and 0.08. From the results, only TiO2 (rutile) and CuO peaks were found. Therefore, when SnO2 is added into the TiO2, no second phases exist, and the SnO2 is solved into the TiO2 matrix as (Ti,Sn)O2. The solid solution effect is shown in Fig. 1(b). The TiO2 (rutile) peak is slightly shifted to the left (low angle side). This is why the lattice parameters of TiO2 unit cell change in response to the solution of SnO2. Based on the result of Bueno’s work [13], the a and c lattice parameters of the TiO2 unit cell slightly increased when the SnO2 was solved into the TiO2 matrix. Therefore, the current systems consist of a mixture of TiO2 and CuO without any second phase. According to this, the present systems seem to be good samples for demonstrating the solid solution effect of SnO2 on the sintering behaviors of a TiO2-CuO system.
3.2 Densification
The TiO2-CuO system was proven to be an effective activated sintering system at low temperature [4, 5]. Moreover, the present experiment was intended to improve densification at a low temperature through a solid solution process. Fig. 2 and 3 show sintered densities at the low temperature of 900°C for the TiO2-CuO system with varying sintering time and SnO2 content. The sintered density was improved up to 0.04 mol of SnO2 content, while the solution effect on the enhancement of density is insignificant at the content of 0.08 mol, compared to the case without SnO2. From the result, there seems to be an optimum amount of solution to enhance the sintered density.
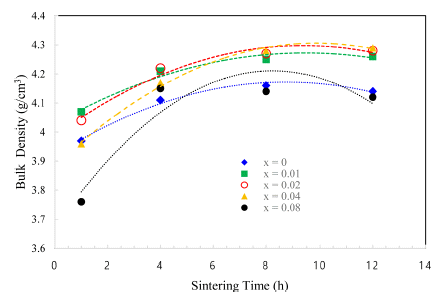
Sintered densities of Ti1-xSnxO2 - CuO(2wt%) samples with sintering time, sintered at 900°C for various contents of SnO2.
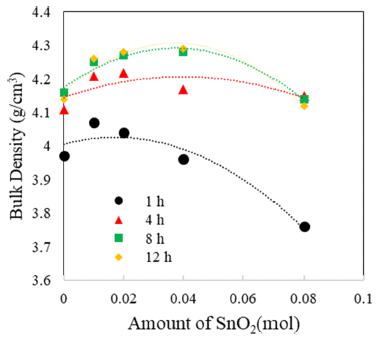
Sintered densities of Ti1-xSnxO2 - CuO(2wt%) samples with SnO2 content, sintered at 900°C for various times.
There are two considerations for the role of dopant as a solid-state sintering aid [14]. First, solid solution effect is operable under the solubility limit. Secondly, second phase effect is workable above the solubility limit. From the X-ray data, it can be seen that there is no second phase in the present samples. Densification was thus affected by the solid solution alone.
In the case of TiO2 systems, densification was improved with the addition of SnO2, according to Bueno’s result [13]. At around 1000°C, a densification mechanism was activated. At the low temperature, volume diffusion as a mass transport mechanism is inactive because pure TiO2 has a high sintering temperature of >1200°C [2, 3]. In the substitution of Ti+4 by Sn+4, oxygen vacancies in the lattice are not created, which is due to the defect chemistry. Instead, Ti interstitials and Ti vacancies can be formed near the grain boundaries. According to this, the grain boundary diffusion can be enhanced by the solid solution process. Therefore, the enhanced grain boundary diffusion can be responsible for the active densification at around 1000°C, as described by Bueno et. al., [13].
Both TiO2-CuO [4, 5] and SnO2-CuO [8, 9] systems are referred to as activated sintering systems. Activated sintering features improved grain boundary diffusion at a low temperature, at which volume(lattice) diffusion is not operable. In both systems, CuO serves as an activator to improve the grain boundary diffusion, and it is responsible for the enhanced densification at a low temperature. However, the amount of activator needed for maximum sintered density is different for each system. The optimum levels of activator for the maximum sintered density are 2 and 0.75wt% for TiO2-CuO [4] and SnO2-CuO [9], respectively. Beyond those optimum levels, the sintered density decreased. Therefore, this study purported a synergy effect of solid solution and activator to increase sintered densities at a low temperature of 900°C for Ti1-xSnxO2-CuO(2wt%). As shown in Fig. 3, the synergy effect was captured with the addition of SnO2. However, the SnO2 content to obtain maximum density was different for a given sintering time. With extended time, the SnO2 content needed for maximum density increased up to 0.04 mol. As a result, the synergy effect described above appears to be significant for improving densification at a low sintering temperature at which lattice diffusion is inactive.
3.3 Microstructure Evolution
Activated sintering features rapid grain growth with the addition of an activator [4, 15]. Fig. 4 shows the SEM microstructure of TiO2-CuO(2wt%) without SnO2. The isolated pores circled inside grains in Fig. 4 are indicative of the rapid grain growth. Fig. 5 shows SEM microstructures of Ti1-xSnxO2-CuO(2wt%). Compared to Fig. 4, pores are mainly located at grain boundaries or junctions rather than inside grains. The trend goes up as the content of SnO2 increased up to 0.04 mol. Accordingly, the rapid grain growth was deterred, and sintered density was enhanced in turn. At x = 0.08, even though pores lie along grain boundaries or junctions, poor densification occurred, as shown in Fig. 5(d).
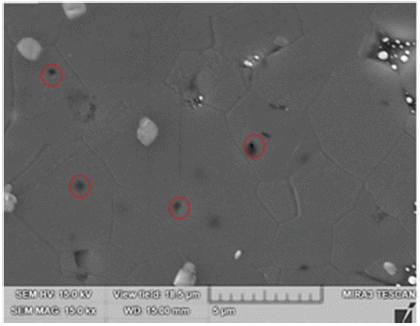
SEM microstructure of TiO2 - CuO(2wt%) sample sintered at 900°C for 12 h. Circles mark isolated pores inside grains. A white phase at grain boundaries or junctions is excess CuO as an isolated particle.
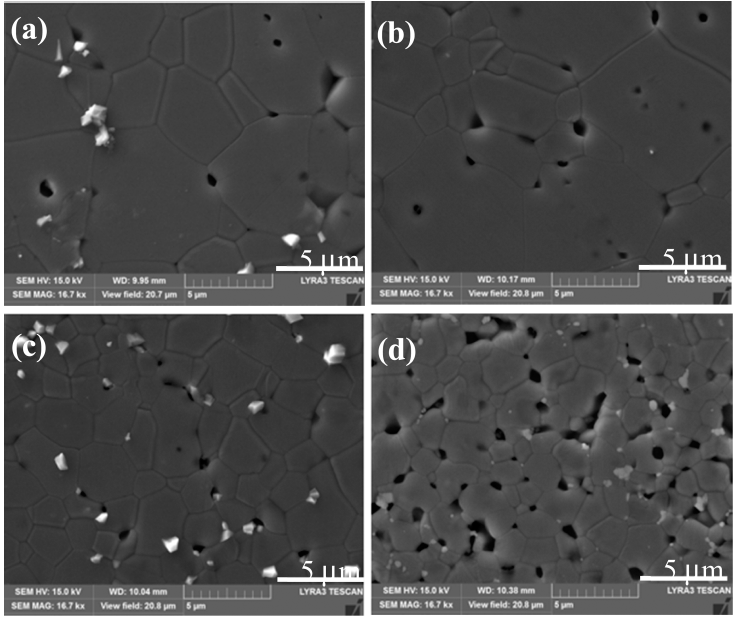
SEM microstructures of Ti1-xSnxO2 - CuO(2wt%) samples sintered at 900°C for 12 h for various SnO2 contents : (a) x = 0.01, (b) x = 0.02, (c) x = 0.04, and (d) x = 0.08. A white phase at grain boundaries or junctions is excess CuO as an isolated particle.
Based on the phase diagram of the TiO2-SnO2 system [13], there is a solid solution range up to 0.08 mol of SnO2 added. However, it is interesting that there is an optimum level of dopant for the sake of density improvement, even within the solid solution range. In consideration of the solid solution effect described by Yan [14], a dopant can change the defect concentration and the surface and grain boundary energies of a matrix material. When the surface and grain boundary energies (γs and γb, respectively) are changed by the dopant, the change in the γb/γs ratio can induce a change in dihedral angles between neighboring grains, such as cos(Φ/2) = γb/2γs ; Φ is the dihedral angle.
In the case of solid-state sintering, when the dihedral angle increases substantially, densification becomes enhanced. In this regard, the present result of the poor densification may result from the decrease in the dihedral angle due to the increase in the γb/γs ratio. Defect creation and change in γb/γs ratio can be competing pathways to improved densification in the current system. That is, the densification improvement by the defect creation could be superior to the decreased densification with the change in γb/ γs ratio up to x = 0.04, while both effects could be reversed at x = 0.08. Nonetheless, the poor densification might be useful for application to a sensor material.
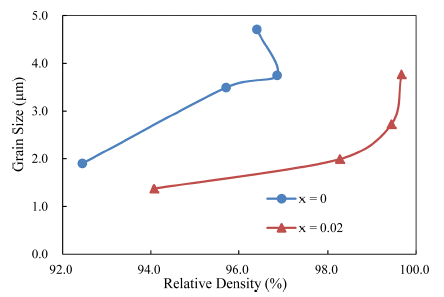
Taking the results together, density versus grain size trajectory is plotted in Fig. 6. To calculate relative density, a theoretical density of 4.294 for TiO2-CuO(2wt%) was used, based on a previous study [4]. At a given density, the grain sizes were smaller in the SnO2-doped samples than in the undoped ones. This means that the synergy effect of solid solution and activator at the low temperature of 900°C for Ti1-xSnxO2-CuO(2wt%) is noticeable for improving densification, but there is an optimum level of the addition of SnO2 for the significant effect.
4. Conclusions
Pure TiO2, as a dielectric material for low-temperature co-fired ceramic (LTCC) technology, has a high sintering temperature of >1200°C. Therefore, it is imperative to reduce the sintering temperature below 900°C. In this context, both CuO as an activator and SnO2 dopant were used to lower the sintering temperature of TiO2. In this investigation, only SnO2 content was varied with the amount of CuO constant. As a result, densification was improved and rapid grain growth was deterred up to 0.04 mol of SnO2. However, at x = 0.08, the solid solution effect of SnO2 is detrimental to densification. This suggests that the synergy effect of solid solution and activator for Ti1-xSnxO2-CuO(2wt%) led to better densification. However, an optimum level of SnO2 dopant exists for the notable effect, even within the solid solution range.
Acknowledgement
This research was supported by a Grant from 2021 Research Funds of Andong National University.