A Study on Synthesis of Ni-Ti-B Alloy by Mechanical Alloying from Elemental Component Powder
Article information
Abstract
A Ni-Ti-B alloy powder prepared by mechanical alloying (MA) of individual Ni, Ti, and B components is examined with the aim of elucidating the phase transitions and crystallization during heat treatment. Ti and B atoms penetrating into the Ni lattice result in a Ni (Ti, B) solid solution and an amorphous phase. Differential thermal analysis (DTA) reveals peaks related to the decomposition of the metastable Ni (Ti, B) solid solution and the separation of equilibrium Ni3Ti, TiB2, and τ-Ni20Ti3B6 phases. The exothermal effects in the DTA curves move to lower temperatures with increasing milling time. The formation of a TiB2 phase by annealing indicates that the mechanochemical reaction of the Ni-Ti-B alloy does not comply with the alloy composition in the ternary phase diagram, and Ti-B bonds are found to be more preferable than Ni-B bonds.
1 Introduction
A solid-state reaction between mixture components during MA is a non-equilibrium process that leads to the formation of metastable phases. The application of MA to exotic systems began after their discovery by Schwarz and Koch [1]. They synthesized the amorphous powders of Ni32Ti68 and Ni45Nb55 by MA starting from a mixture of pure crystalline powders. The amorphization is attributed to a solid-state interdiffusion reaction, the kinetics of which is controlled by the excess point and lattice defects generated by plastic deformation. The accumulation of point and lattice defects by plastic deformation raises the free energy of the faulted intermetallic above that of the amorphous alloy. Since then the formation of one or more phases using MA has attracted considerable attention. Using this technique, a number of studies have been carried out to synthesize a range of stable and metastable phases including supersaturated solid solutions, crystalline and quasi-crystalline intermediate phases, as well as amorphous alloys [2-4]. Many studies on the synthesis of Ni-based alloys by MA have been reported. In particular, binary Ni-(B,Ti) systems have been shown to form the solid solution and exhibit alloying behavior from the mixture components [5-7].
On the other hand, in a ternary Ni-Ti-B system, the alloys have been prepared by a liquid solidification method, such as melt spinning, arc melting and suction casting. Several studies have examined amorphous materials produced by melt spinning were studied [8, 9]. Although the Ni-based composite coating such as Ni-TiB2 exhibits excellent wear resistance [10], researches connected with the mechanochemical synthesis and crystalline of ternary Ni-Ti- B alloy obtained by means of MA are not found in literature. In a Ni-Ti-B system using MA, the structure of the resulting product is expected to be determined by the competitive parameters of Ni-Ti, Ni-B and Ti-B pairwise interactions. The objective of the present work is to study the synthesis of Ni-Ti-B alloy powder by MA, as well as analysis of its structure and phase transitions during the crystallization of ternary alloys.
2 Experimental
The Ni82Ti12B6 alloy powder was synthesized from elemental components of nickel powder (99.9%), titanium powder (99.9%) and boron powder (99%) with a particle size 2~3 μm, 100 μm and 45 μm, respectively. The powder mixture was loaded into SUS304 steel vials with SU2J bearing steel balls. A ball to powder ratio (BPR) of 50:1 was maintained under an inert argon atmosphere to prevent oxidation. MA process was performed using vibratory ball mill machine, which agitates the vials at 25Hz with an amplitude of 2.5~3.0 mm. The vials were rotated every hour to favor the fracture of particles and minimize the formation of ductile coatings on the walls and balls. The mechanical alloyed powder and subsequent annealed powder was investigated by X-ray diffraction (XRD, RIGAKU D/MAX) using Cu Kα-radiation. The thermal transition behavior was analyzed by differential thermal analysis (DTA, DTG-60H) under non-isothermal conditions at a heating rate of 10°C/min with flowing argon, and the temperature ranged from 100°C to 1000°C. To confirm the DTA curves, the as-milled powder after 60 h was annealed in a furnace at 350°C, 410°C and 500°C at a heating rate of 10°C/min under an argon atmosphere.
3 Result and Discussions
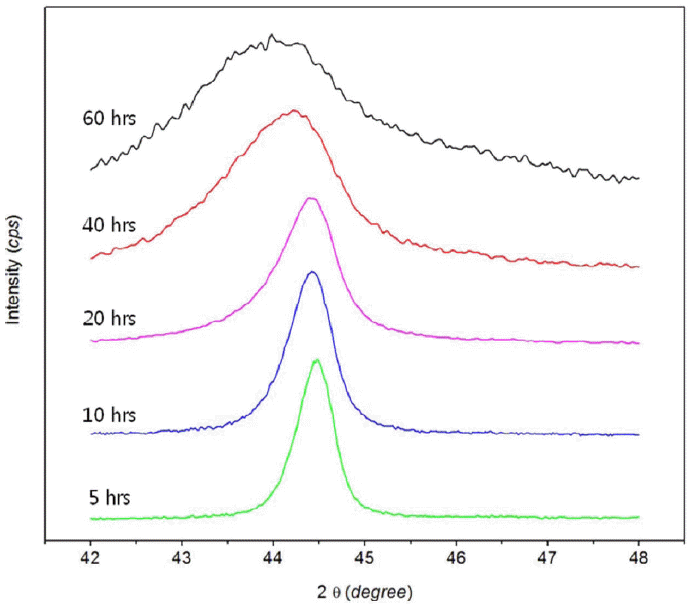
Fig. 1 presents XRD patterns of the Ni82Ti12B6 powder after different milling times. The as-received powder showed peaks related to Ni and Ti without B due to the main difference between the boron intensity and others peaks. No phase transformation was detected until 60 h milling, but the peaks related to Ti disappeared 40 h of milling. Further milling up to 60 h did not result in the formation of any other intermetallics, and became an amorphous. On the other hand, the solid state interaction in the Ni82Ti12B6 system led to the formation of a Ni (Ti, B) FCC solid solution, as confirmed by the results from XRD (Fig. 2). The phase shifted to the minor angles compared to the position of the Ni (111) peak. This suggests that the mechanochemical reaction between the components of the mixture resulted in the formation of a Ti and B solid solution in FCC Ni. Fig. 3 presents the change in the Ni lattice parameter and microdeformation root-meansquare (εrms) of the lattice for FCC phase of the Ni82Ti12B6 alloy on MA time. The lattice parameter and εrms of Ni increased continuously with increasing milling time due to the interstitial diffusion of Ti, B into the Ni lattice. The lattice parameter of the Ni (Ti, B) solid solution (a=0.35630 nm) was considerably higher than that of the Ni (B) equilibrium solid solution containing 15 wt.% B (a=0.35255 nm [6]). In addition, the present study showed that the εrms value ( 1.1%) of Ni lattice was significantly increased in comparison with the εrms of Ni (B) solid solution (εrms ≃ 0.48 [11]). If only B dissolves in the Ni lattice during the chemical interaction of the components and Ti remains as a second amorphous phase, then according to [6], the lattice parameter of the Ni (B) solid solution in this study should be lower than 0.35255 nm. These results indicate that the higher lattice parameter and εrms of the FCC Ni phase can be explained by the supplementary solid solution of Ti in Ni. In addition, the defective region caused by microdeformation in the solid solution lattice can contain enhanced concentrations of Ti and B atoms as elements in the non-equilibrium FCC lattice.
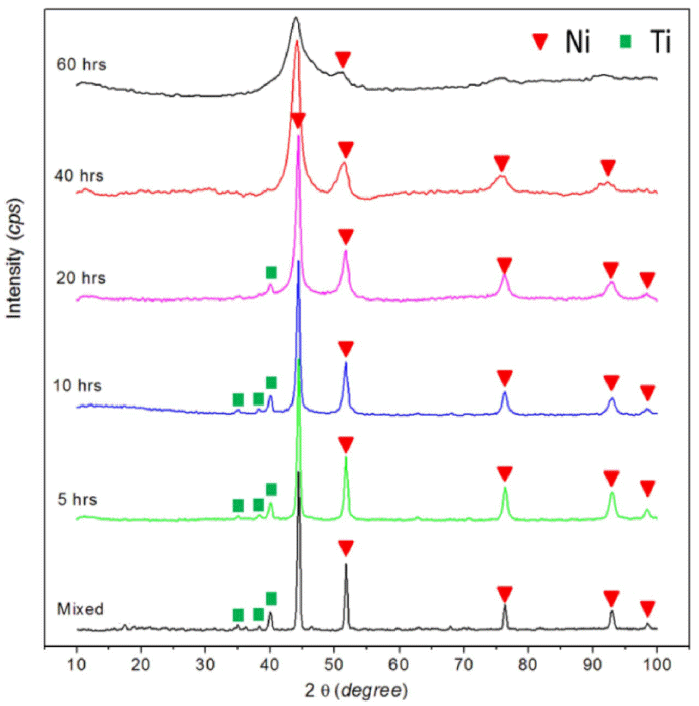
XRD patterns of Ni82Ti12B6 initial mixture of components and alloyed powder after different milling time.
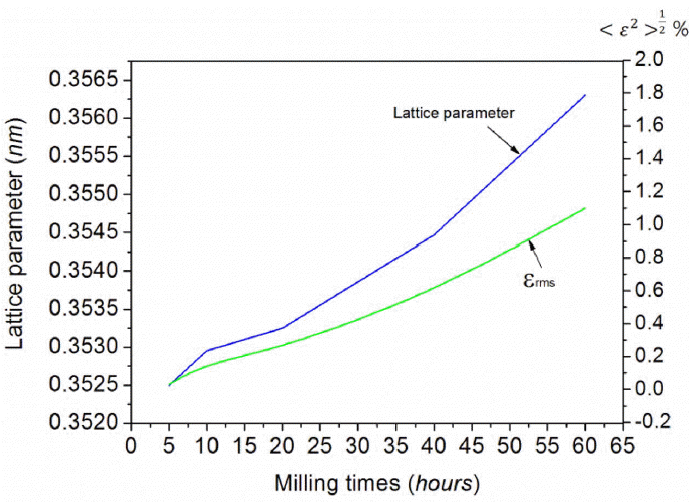
Change in the Ni lattice parameter and root-meansquare microdeformation for the lattice of the fcc phase of the Ni82Ti12B6 alloy on MA time.
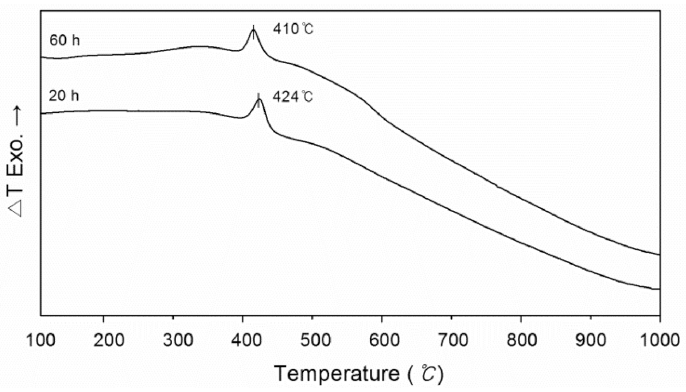
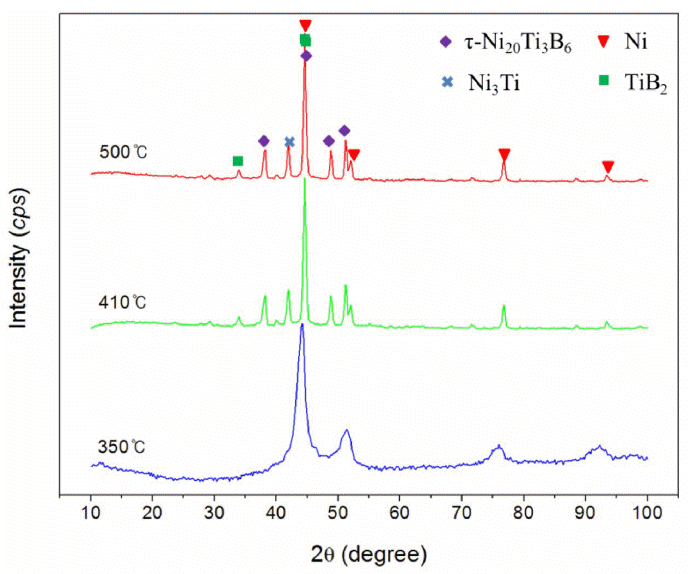
DTA characterization of MA powder during heating attributed the heat effects to structural changes in the metastable alloys. DTA (Fig. 4) revealed only one exothermic peak on the MA powder for 20 h and 60 h, respectively, in a narrow temperature range 400~430°C. This was attributed to decomposition of the metastable Ni (Ti, B) solid solution and to separation of the equilibrium Ni3Ti, TiB2 and τ-Ni20Ti3B6 phases, which was confirmed by XRD of the annealed powder, as shown in Fig. 5. No phase transformation was observed after heating of powder up to 350°C, and the peaks of the FCC Ni phase were sharper. After annealing up to 410°C, considerable peaks related to Ni3Ti, TiB2 and τ-Ni20Ti3B6 phases were detected. Therefore, until thermal decomposition of the solid solution, the Ni (Ti, B) solid solution is energetically more stable. DTA showed that the peak temperatures with the formation of the above mentioned intermetallics decreased from 410°C to 424°C with increasing milling time. This observation can be explained by considering the energy given to the milled powder during MA, which can help the transformation to run at lower temperature. Thus, with increased milling times, more energy is transferred to the powder [6].
In the FCC lattice of Ni, Co and γ-Fe, Goldschmidt considered the possibility of isostructural metastable cluster groups of Metal and B atoms similar to the formation of Genie-Preston zones (G-P zone). B atoms penetrate into the FCC Ni lattice, which leads to strong distortion of its lattice and these clusters are stable up to the moment when the boride phase with another structure separates from the solid solution [12-14]. In particular, the formation of boride phases in Me-B bonds is agreement with Hägg’s dimensional rule formulated for the metal-metalloid systems. This rule states that amorphous alloys can be formed only if the ratio of constituent’s atoms radii satisfies the inequality, Fig. 6
where the lower limit comes from the geometric constraints in dense packing structures, whereas the upper limit is an empirical value derived from the early experiments on rapid quenching [15]. In previous work, Me-B bonds depended substantially on the Hägg’s ratio rB/rMe and were maximal for metals, i.e. 0.59 [13]. These ratios were 0.61 for Ti and 0.72 for Ni, respectively, which suggested that the formation of Ti-B bonds was more preferable than Ni-B bonds. This result was confirmed from XRD analysis in Fig. 5 showing the phase compositions of the powder after annealing. Thus, the peaks related to Ni3Ti, TiB2 and τ-Ni20Ti3B6 phases were detected, while nickel boride phases (Ni3B, Ni2B, Ni4B3 and NiB) were not formed after annealing up to 500°C.
It was worth noting that the τ phase was formed from the solid solution Ni (Ti, B). The τ phase was a suspected intergranular phase in the TiB2-Ni composites tested for wear applications, which has a FCC-Cr23C6 (τ-phase) structure and was reported by Schöbel and Stadelmaier [16]. This was manufactured using the Czochralski growth method [17] and SHS (self-propagating high temperature synthesis) [18], but the formation of a τ-phase by MA has not been described. In addition, according to the equilibrium phase diagram [19], although TiB2 phase is not contained in the composition of Ni82Ti12B6 alloy, being disintegrated from solid solution after annealing.
The formation of stable crystalline phase by homogeneous nucleation depended strongly on the glass-crystal interface energy, which could be interpreted largely in terms of the entropy charge at the interface. On the other hand, based on the clustered short range order model such as G-P zone, nucleation was less sensitive to the structure of the glass-crystal interface and presumably more dependent on the precise alloy composition [8]. In present study, the formation of crystalline phases was expected to follow the alloy composition because of the clusters in the solid solution in the Ni lattice. However, the result of this study showed that the mechanochemical reaction in the Ni-Ti-B alloy by MA does not comply with the alloy composition in the equilibrium phase diagram.
4 Conclusions
The mechanochemical synthesis of a Ni82Ti12B6 alloy by vibratory ball milling from individual components resulted in the formation of a Ni (Ti, B) solid solution, which led to a significant increase in the lattice parameter and microdeformation in lattice of the FCC Ni. The Ni (Ti, B) solid solution was stable until the separation of boride phases as a result of heat treatment up to 410°C. After annealing, Ni3Ti, TiB2 and τ-Ni20Ti3B6 phases with stable chemical bonds were observed but no nickel boride phases were found, because B in the Ni lattice contributed to the formation of titanium diboride and ôphase. This phenomenon was explained by Hägg ratio which predicts the formation of an amorphous alloy. Therefore, Hägg’s dimensional rule had a considerable effect on the competitive parameter of Ni-B and Ti-B pairwise interactions.