A Study on the Recovery of Li2CO3 from Cathode Active Material NCM(LiNiCoMnO2) of Spent Lithium Ion Batteries
Article information
Abstract
In this study, an experiment is performed to recover the Li in Li2CO3 phase from the cathode active material NMC (LiNiCoMnO2) in waste lithium ion batteries. Firstly, carbonation is performed to convert the LiNiO, LiCoO, and Li2MnO3 phases within the powder to Li2CO3 and NiO, CoO, and MnO. The carbonation for phase separation proceeds at a temperature range of 600°C~800°C in a CO2 gas (300 cc/min) atmosphere. At 600~700°C, Li2CO3 and NiO, CoO, and MnO are not completely separated, while Li and other metallic compounds remain. At 800 °C, we can confirm that LiNiO, LiCoO, and Li2MnO3 phases are separated into Li2CO3 and NiO, CoO, and MnO phases. After completing the phase separation, by using the solubility difference of Li2CO3 and NiO, CoO, and MnO, we set the ratio of solution (distilled water) to powder after carbonation as 30:1. Subsequently, water leaching is carried out. Then, the Li2CO3 within the solution melts and concentrates, while NiO, MnO, and CoO phases remain after filtering. Thus, Li2CO3 can be recovered.
1 Introduction
The cathode active material NMC(LiNiCoMnO2) of waste lithium ion batteries is one of the compounds of the cathode active materials of various lithium secondary batteries. The materials of the ternary system have the same structure as LiCoO2 and capacity per volume and the operating voltage is similar to LiCoO2 and has low content of Co, so the price benefit is good and a stable cycle is possible inside the high voltage domain [1].
Small home appliances and mobile phones mainly use LiCoO2 (LCO), but this is unsuitable to be used in medium/large batteries, which require a large output, due to the price rise of cobalt and danger of explosion. So, ternary cathode active material, which is a cathode material to replace LCO, which uses a lot of high-priced cobalt, has been highlighted. It mainly consists of three metals, nickel, cobalt, and manganese. Currently, Li(NixCoyMnz)O2 (NCM) has been applied as a high capacity cathode material for hybrid electric vehicles (HEV). For the cathode material of the NCM system for electric cars, which is expected to be produced in earnest, the necessity for recycling and reprocessing scrap is on the rise [2-5].
Many studies on how to recover the valuable metals from the cathode of waste lithium ion batteries have been performed.
In research at the beginning of the 2000s, Lee and Kim studied the leaching behavior of nitric acid or sulfuric acid to recover lithium and cobalt from LiCoO2, which is the cathode material separated from waste lithium ion batteries, and how to improve the leaching rate. They used reducing agents such as Na2S2O3 and H2O2 and investigated the optimal leaching conditions of LiCoO2 [10]. In addition, in 2001, Lee and Kim studied the behavior of reduction leaching of LiCoO2 in sulfuric acid solution in detail and established conditions for the concentration of sulfuric acid, leaching temperature, density of the beginning pulp, dosage of hydrogen peroxide and reaction temperature [9].
In 2005, Swalin et al. leached LiCoO2 into sulfuric acid and used a solvent extraction method and did research to recover more than 99.99% of the cobalt solution from sulfuric acid. In 2010, Lee and Chung5) leached LiCoO2 into sulfuric acid, separated and refined it for solvent extraction, used an alkaline reagent, and produced more than 99.98% of cobalt trioxide through cobalt hydroxide and heat treatment. They also performed research to manufacture lithium by using sodium carbonate [7]. Also, for the NCM system, Kim et al. produced a precursor of cathode active material of nickel, cobalt, and manganese through physical/chemical treatment of waste lithium ion battery packs of electric cars. Using a coprecipitated filtrate, they performed a study to make lithium carbonate [17]. However, for the recycling method of cathode active material of the original secondary battery, valuable metals such as cobalt, nickel, and manganese are recovered and lithium is recovered in the form of lithium carbonate by using sodium carbonate. In this case, sodium is an impurity and the fact that many washing processes are required is a disadvantage.
This research studied the pyrometallurgical recovery process of waste lithium battery cathode material in the NCM system that is eco-friendly and can obtain the recovery of material with high purity with simple processing for Li metal to be recovered in the waste lithium battery powder in the NCM system. Following heat treatment in a reducing atmosphere(mixed atmosphere of CO and CO2 or independent atmosphere of CO2), after isolating the phases of Li, Ni, Mn, and Co, the recovery of lithium was performed through water leaching by using the solubility difference of Ni, Co, and Mn.
2 Experimental
2.1 Experiment Materials
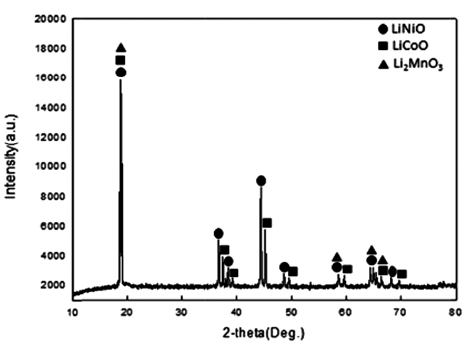
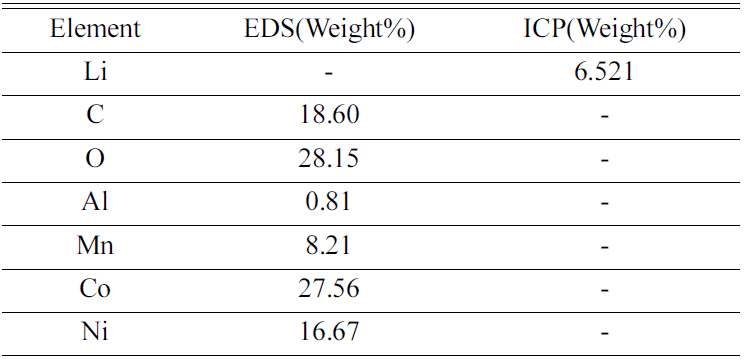
Cathode active material NMC(LiNiCoMnO2) powder of waste lithium ion batteries was used in the experiment. The chemical composition of the experiment materials was analyzed with X-ray diffraction (XRD) and energy-dispersive X-ray spectroscopy (EDS) and three phases in the forms of LiNiO, LiCoO, and Li2MnO3 were composed. The XRD results are shown in Fig. 1, and the EDS results are shown in Table 1. The lithium content of experiment materials was measured with ICP analysis, and we confirmed that it was composed of 6.521% lithium. The results are shown in Table 1.
2.2 Carbonation
To extract Li from the cathode active material NMC (LiNiCoMnO2) powder of waste lithium ion batteries, carbonation was performed to separate Li and Ni, Mn, and Co. An electric furnace was used for the experiment apparatus, and the scheme for that is shown in Fig. 2. Using an SUS crucible, a white lubricated release agent was spread to prevent the sample from being absorbed into the crucible. A 30g sample was used.

Schematic diagram of experimental apparatus for carbonation of NCM system waste Li-ion battery powder.
For the experiment temperature, the heating rate was set as 10°C/min and it was maintained for 2 hours at 600°C, 700°C, and 800°C and then reduced by 10°C/min. An Ar atmosphere was used during heat rising and CO2 atmosphere in the maintenance step after heat rising, and CO2 gas was blown in at 300cc per minute. At the stage of heat reduction after maintenance, the Ar atmosphere was maintained again.
2.3 Water Leaching
After heat treatment with CO2, the experiment was performed by pulverizing the battery powder of waste lithium batteries in the NCM system, which was sintered. By using the solubility difference of Li2CO3 and metallic oxides (NiO, MnO, CoO), an experiment to separate Li2- CO3 and metallic oxides (NiO, MnO, CoO) through water leaching was performed. For experiment apparatus, a mixer and beaker were used. Distilled water was usedas the solvent for water leaching, and the ratio of distilled water to NMC(LiNiCoMn) powder of the cathode active material of waste lithium ion batteries was set to be 30:1. The time for water leaching was 5 hours.
3 Result and Discussion
3.1 Carbonation
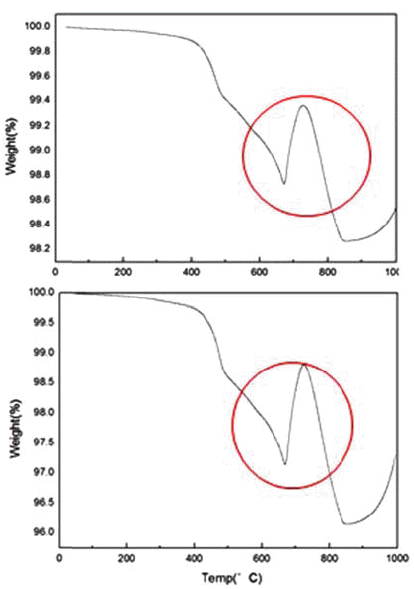
First, the thermal behavior of cathode active material NMC(LiNiMnCoO2) of waste lithium batteries was confirmed with a thermogravimetric apparatus (TGA) and the heat rising temperature was 995°C/min and heat rising speed was 5°C/min, and they were measured in a CO2 atmosphere. The results are shown in Fig. 3.
As a result of TGA analysis, a big weight change was observed between 650°C and 800°C and the phase change occurred in this temperature range.
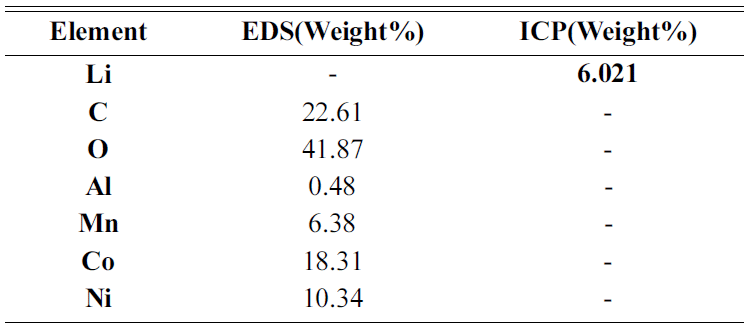
In the TGA experiment results, the battery powder of the NCM system carbonated at 600°C, 700°C, and 800°C and to see the phase change during the process, XRD analysis was performed. The XRD analysis results are shown in Fig. 4. Looking at the XRD analysis results, when carbonation was performed at 600°C and 700°C, Li2CO3 and NiO, CoO, and MnO were not completely separated, and Li and the compounds of other metals remained. It was confirmed that the phase of the raw sample, which was LiNiO, LiCoO, and Li2MnO3, was completely separated into NiO, CoO, and Li2CO3 phases by carbonation at 800°C. The Chemical composition after Carbonation is shown in Table 2. It was observed that lithium content was not changed during thermal treatment.
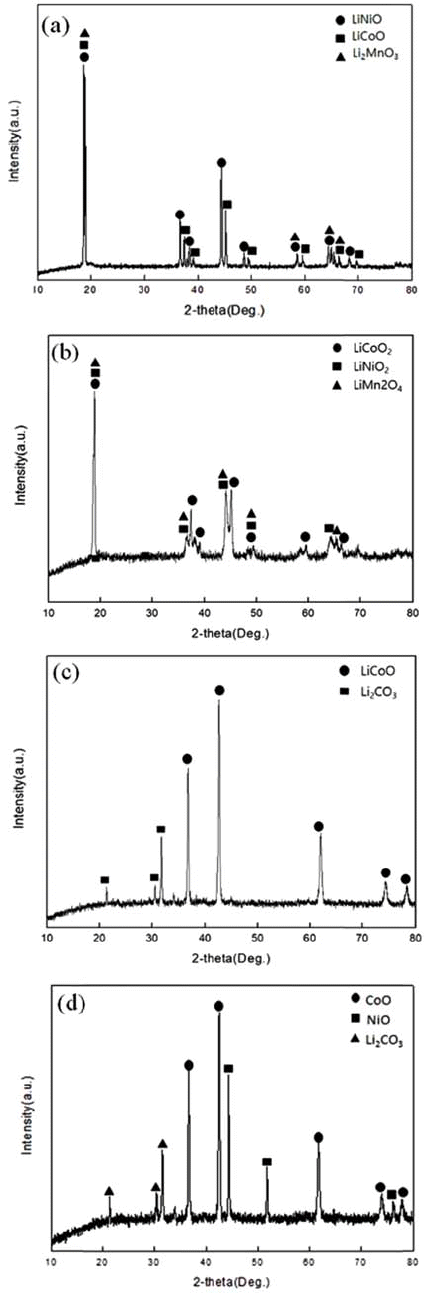
XRD patterns of (a) Raw sample (b) After carbonation at 600°C (c) After carbonation at 700°C (d) After carbonation at 800°C.
3.2 Water Leaching
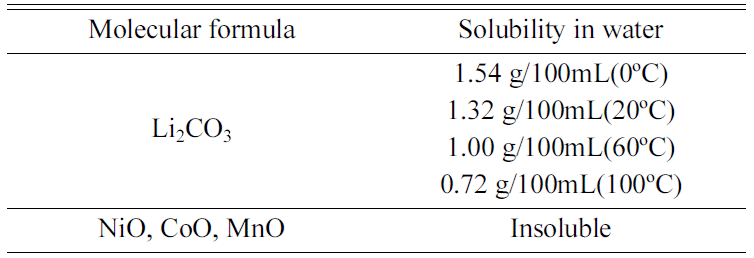
After pulverizing the sintered sample after carbonation, water leaching was performedby using the solubility difference of Li2CO3 and Ni, CoO phases. Table 3. shows the solubility difference according to the temperature of Li2CO3 and Ni, CoO phases.
In Table 3, in the case of Ni and CoO, they do not melt in water, and in the case of Li2O3, because solubility decreases as temperature rises, flushing at a lower temperature is more favorable.
If we consider that Li content in the raw sample is 6.52wt.% and the analyzed solution is 10 ml, when the ratio of the weight of distilled water to powder after carbonation is 30:1, we can predict that Li2CO3 in the sample after carbonation was completely dissolved in water.
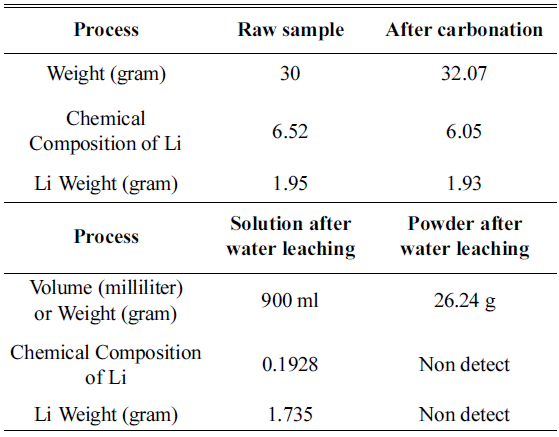
During water leaching, the ratio of distilled water to NMC sample after carbonation was set as 30:1 and was done for 5 hours, and powder and solution were separated through a decompressed filter after water leaching. The phase change during water leaching of dried powder after filtering was observed through XRD analysis, and the resultsare shown in Fig. 5. Also, to confirm the Li2- CO3 amount dissolved in the solution, ICP of powder and ICP analysis of the solution were performed, and the results are shown in Table 4.
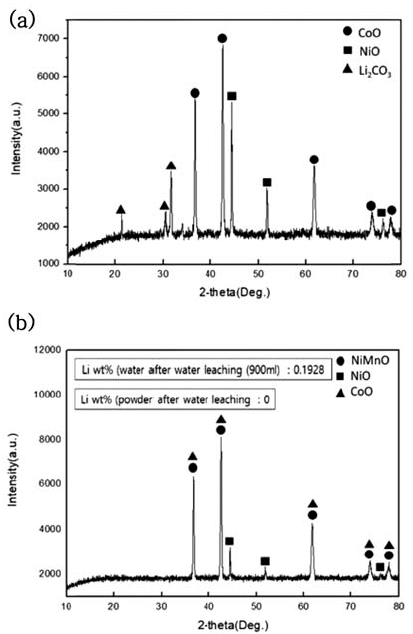
XRD patterns of NMC system waste Li-ion battery powders after water leaching. (a) After carbonation at 800°C (b) Powder After water leaching

Weight change of sample and change of Li content in the overall process and content of Li in the process
When the water leaching of NMC powder after carbonation was processed, Li2CO3 phase was all dissolved in the solvent and concentrated and we confirmed that only NiO, CoO, and NiMnO phases remained in the powder after the decompressed filter
3.4 Li Behavior during the Overall Process
Along with the results of the weight change of the sample during the overall process and ICP analysis results, Li content by step is shown in Table 4.
Powder after water leaching and lithium content in the solution after water leaching were calculated and are shown in Table 4. The ratio of distilled water to powder after carbonation was set to be 30:1, and after water leaching for 5 hours, 1.735 g of Li was dissolved in NMC powder after carbonation and we confirmed that it was completely dissolved in water without Li2CO3 and concentrated in the solution. Based on the results, when leaching three times ata 10:1 ratio or water leaching of distilled water to sample at a ratio of 30:1 was processed, we confirmed that Li2CO3was completely dissolved and concentrated in the solution.
4 Conclusions
Though phase separation and water leaching followed by carbonation of the cathode active material NMC(LiNi- CoMn) powder of waste lithium ion batteries, the following results for Li2CO3 recovery after separating Li2CO3 and metallic oxides (NiO, MnO, CoO) were obtained.
1) The phase separation for waste lithium battery powder in the NCM system in CO2 gas atmosphere through carbonation occurred at 650°C~800°C and the phase separation of Li2CO3and metallic oxides (NiO, MnO, CoO) occurred.
2) Only some had phase separations at 600°C, 700°C and 800°C, and there was phase separation of three phases such as LiNiO, LiCoO, and Li2MnO3 into NiO, CoO, and Li2CO3 phases after CO2carbonation.
3) By using the solubility difference of Li2CO3and NiO, CoO, and MnO phases, water leaching was processed and the ratio of distilled water to NMC powder after carbonation was set to be 30:1 and was done for 5 hours. When a decompressed filter was used, Li2CO3 was completely dissolved and concentrated in the solution. NiO, MnO, and CoO phases remained inside the powder after filtration and Li2CO3 and metallic oxides (NiO, MnO, CoO) were completely separated.
Acknowledgement
This study was supported by the R&D Center for Valuable Recycling(Global-Top R&BD Program) of the Ministry of Environment.(Project No.:2016002220001) and was also supported by the BB21+ Project in 2018.