Exploring Thermoelectric Transport Properties and Band Parameters of n-Type Bi2-xSbxTe3 Compounds Using the Single Parabolic Band Model
Article information
Abstract
The n-type Bi2-xSbxTe3 compounds have been of great interest due to its potential to achieve a high thermoelectric performance, comparable to that of p-type Bi2-xSbxTe3. However, a comprehensive understanding on the thermoelectric properties remains lacking. Here, we investigate the thermoelectric transport properties and band characteristics of n-type Bi2-xSbxTe3 (x = 0.1 – 1.1) based on experimental and theoretical considerations. We find that the higher power factor at lower Sb content results from the optimized balance between the density of state effective mass and nondegenerate mobility. Additionally, a higher carrier concentration at lower x suppresses bipolar conduction, thereby reducing thermal conductivity at elevated temperatures. Consequently, the highest zT of ~ 0.5 is observed at 450 K for x = 0.1 and, according to the single parabolic band model, it could be further improved by ~70 % through carrier concentration tuning.
1. Introduction
Thermoelectric (TE) technology directly converting waste heat into electricity holds significant promise in environmental protection [1, 2]. As the efficiency of thermal-to-electrical energy conversion relies heavily on TE materials, extensive research efforts have been devoted to enhancing TE performance and developing new materials over the past 60 years [3-6]. Bismuth telluride (Bi2Te3)-based materials, including (Bi,Sb)2Te3 for p-type and Bi2(Te,Se)3 for n-type, have long been recognized for their significant potential in commercial thermoelectric applications, owing to high TE conversion efficiency [7-9]. The materials’ TE performance is evaluated by a dimensionless figure of merit zT=S2σT/κtot (κe+κL), where S, σ, T, κtot, κe and κL are the Seebeck coefficient, electrical conductivity, absolute temperature, total thermal conductivity, electronic thermal conductivity, and lattice thermal conductivity, respectively [10] While zT value serves as a primary parameter, balancing between the p- and n-type materials also plays a pivotal role in determining the overall performance of a TE device [11]. Over the decades, efforts to improve zT of TE materials have led to notable advancements achieved through a diverse array of strategies including nanostructuring [12-14], lattice planification [15], carrier filtering [16, 17], resonant doping [18, 19], band convergence [20, 21] and so forth. However, the TE performance of n-type Bi2(Te,Se)3 compounds (with zT ~1.22 at 323K) [22] lags somewhat behind that of their counterpart p-type (Bi,Sb)2Te3 (with zT ~1.86 at 320K) [23], posing challenges in the development of high-performance TE devices and thereby limiting their practical applications.
The inferior TE performance of n-type Bi2(Te,Se)3 originates from intrinsically unfavorable electronic transport properties, such as high carrier concentration [24], strong dependence of carrier mobility on structural texturing [25], and single valley contribution to electrical properties of Bi2Se3 [26]. Recently, Zhou et al. developed a new n-type Bi2-xSbxTe3, tackling the aforementioned issues in conventional n-type Bi2(Te,Se)3 [11]. It offers several advantages including optimized electron concentration, multi-valley contribution for high S, and larger mass differences for low κ, resulting in a high zT value of ~1.0 at 330 K for Bi1.5Sb0.5Te3.0 even without applying strategies to enhance TE properties [11]. This highlights the possibility of further improving the zT of n-type Bi2Te3-based materials. In this regard, a comprehensive investigation into the TE transport properties and understanding of the electronic band structure of n-type Bi2-xSbxTe3 is necessary as a preliminary study.
In this work, we investigated the temperature-dependent TE transport properties of n-type Bi2-xSbxTe3 (x = 0.1 – 1.1) and characterized the band parameters using the single parabolic band (SPB) model. The calculated weighted mobility (μw) was found to be highest at x = 0.1 and decreased with increasing Sb content, indicating that lower Sb content led to more favorable electronic transport properties. Consequently, a maximum power factor (S2σ) of ~2.2 mW m-1 K-2 was observed at 300 K for x = 0.1. Meanwhile, the higher carrier concentration at lower x suppressed the bipolar contribution to the total thermal conductivity (κtot). As a result, the peak zT value for x = 0.1 was the highest, reaching ~ 0.5 at 450 K. Furthermore, SPB model-based estimation of Hall carrier concentration (nH) dependent zT values suggests that the zT value for x = 0.1 could be improved by ~70% (from 0.3 to 0.5) through optimizing nH at room temperature.
2. Experimental Procedure
Bi2-xSbxTe3 (x = 0.1, 0.3, 0.5, 0.7, 0.9, 1.1) powders were synthesized via a ball-milling process. High-purity (~99.999%) Bi, Sb, and Te shots were used as raw materials. They were weighed according to the desired ratio to achieve stoichiometry and ball-milled using planetary mill (Pulverisette 5, Fritsch, Germany). The ball-milling process consisted of two steps: the first step operated at 360 rpm for 48 cycles, and the second step operated at the same speed for 24 cycles. The obtained powder was reduced in the H2 atmosphere at 573K in 90 min. Cylinder-type bulks (15 mm in diameter and 12 mm in thickness) were fabricated by using spark plasma sintering (SPS) at 753K for 5 min under 30, 40, and 50MPa. The crystalline phases of the ball-milled powders were identified using X-ray diffraction (XRD) with a Cu Kα1 X-ray source (Malvern Panalytical, United Kingdom). The temperature-dependent electrical conductivity and Seebeck coefficient were measured by SBA458 Nemesis (Netzsch, Germany) from 298K to 473K in an argon atmosphere. The Hall carrier concentration and Hall mobility were measured using the van der Pauw configuration with an HMS3500 (Ecopia, South Korea) at room temperature. The temperature-dependent thermal conductivity was determined using the equation of κ = DρCp, where D, ρ and Cp are thermal diffusivity, density and specific heat. Thermal diffusivity was measured by laser flash method (NETZSCH, Germany) and the density was measured using Archimedes method. The specific heat was estimated by the corrected Dulong-Petit law [27, 28]. The electrical and thermal properties were measured along the direction perpendicular to the pressing direction of SPS.
3. Results and Discussion
Fig. 1(a) and Fig. 1(b) shows the X-ray diffraction (XRD) patterns of n-type Bi2-xSbxTe3 (x = 0.1 – 1.1) ball-milled powders. The peaks are clearly indexed to Bi2Te3 (JCPDS #15-0863), indicating the synthesis of single phases of n-type Bi2-xSbxTe3 (x = 0.1 – 1.1) across all compositions. The peaks shifted to higher angles with increasing x, suggesting the change in lattice parameters upon the substitution of Sb at Bi sites. As illustrated in Fig. 1(c), the calculated lattice parameter a gradually decreased with increasing Sb content, which can be attributed to the smaller atomic size of Sb compared to Bi (rSb = 1.45 Å, rBi = 1.60 Å). Meanwhile, it was observed that the lattice parameter c increased, likely due to the differences in electronegativity between Bi and Sb [11] These results suggest that the successful substitution of Sb atoms at the Bi site without the formation of any second phases.
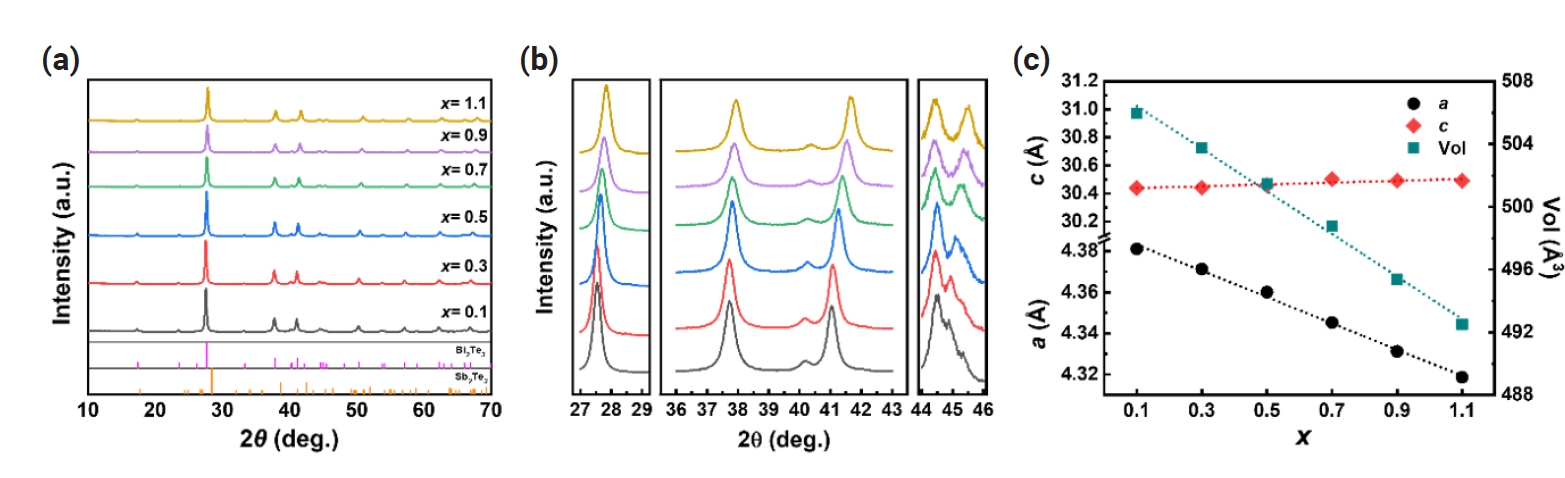
(a) X-ray diffraction (XRD) patterns and (b) three close-up views of (a) with the 2θ ranging between 27–29°, 36–43° and 44–46° (c) lattice parameters (a and c) and unit cell volume of n-type B2-xSbxTe3 (x = 0.1 – 1.1).
Fig. 2 shows the electrical conductivity (σ), Seebeck coefficient (S), and power factor (S2σ) as a function of temperature for spark plasma sintered (SPSed) Bi2-xSbxTe3 (x = 0.1 – 1.1). As shown in Fig. 2(a), Bi1.9Sb0.1Te3 exhibited the highest σ at room temperature, ~1895 S cm-1, and decreased with increasing temperature to ~1093 S cm-1 at 473K. The σ of the Bi2-xSbxTe3 compound gradually decreased as the x decreases due to the suppression of donor-like effect by excess BiTe antisites defect, with the lowest σ of ~237 S cm-1 observed at room temperature for x = 1.1. The measured S values are negative, indicating n-type carrier transport behavior. The absolute value of S increased with increasing x from 108 μV K-1 (x = 0.1) to 148 μV K-1 (x = 1.1) at 300 K (Fig. 2b). Also, it is observed that the temperature at which the peak S value occurs shifted to higher temperatures with decreasing Sb content. The changes in σ and S can be ascribed to the trade-off relationship between σ and S, and the differences in temperature dependence of S with Sb content are due to the increased or reduced bipolar contribution at different x [29-33]. The power factor (S2σ) calculated from the measured σ and S exhibited the highest value of ~2.2 mW m-1 K-2 for x = 0.1 and gradually decreased with increasing x, and reaching ~0.5 mW m-1 K-2 for x = 1.1 at 300K (Fig. 2c).
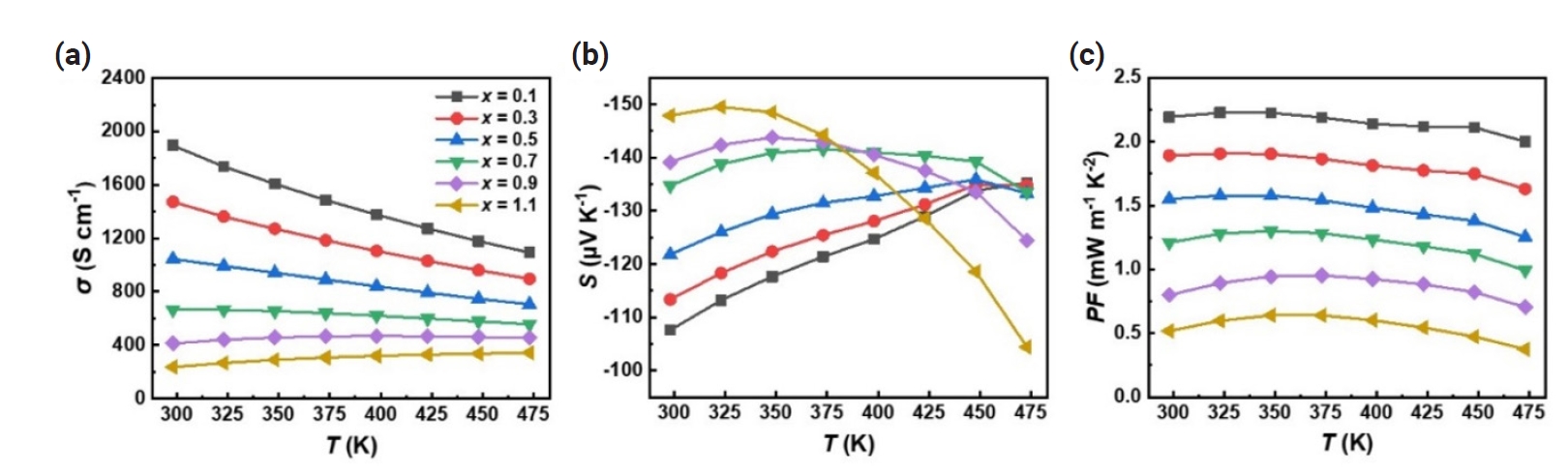
Temperature-dependent (a) electrical conductivity (σ), (b) Seebeck coefficient (S), and (c) power factor (S2σ) of spark plasma sintered (SPSed) Bi2-xSbxTe3 (x = 0.1 – 1.1).
To understand the change in electrical properties, we measured the Hall carrier concentration (nH) and Hall mobility (μH), as shown in Table 1. As expected, both nH and μH gradually decreased with increasing x, which accounts for the decrease in σ and the increase in S with increasing Sb content. To gain a deeper understanding on the origin of differences in electronic transport properties of Bi2-xSbxTe3 (x = 0.1 – 1.1), we estimated the density-of-state effective mass (md*) and nondegenerate mobility (μ0) using the single parabolic band (SPB) model. We obtained nH dependent S (nH-S) curve for a given md* using the following equations and fitted them to the experimentally measured nH and S (Fig 3a),
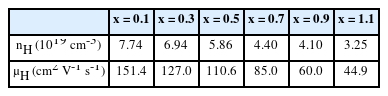
Hall carrier concentration (nH) and Hall mobility (μH) of Bi2-xSbxTe3 (x = 0.1 – 1.1) measured at room temperature
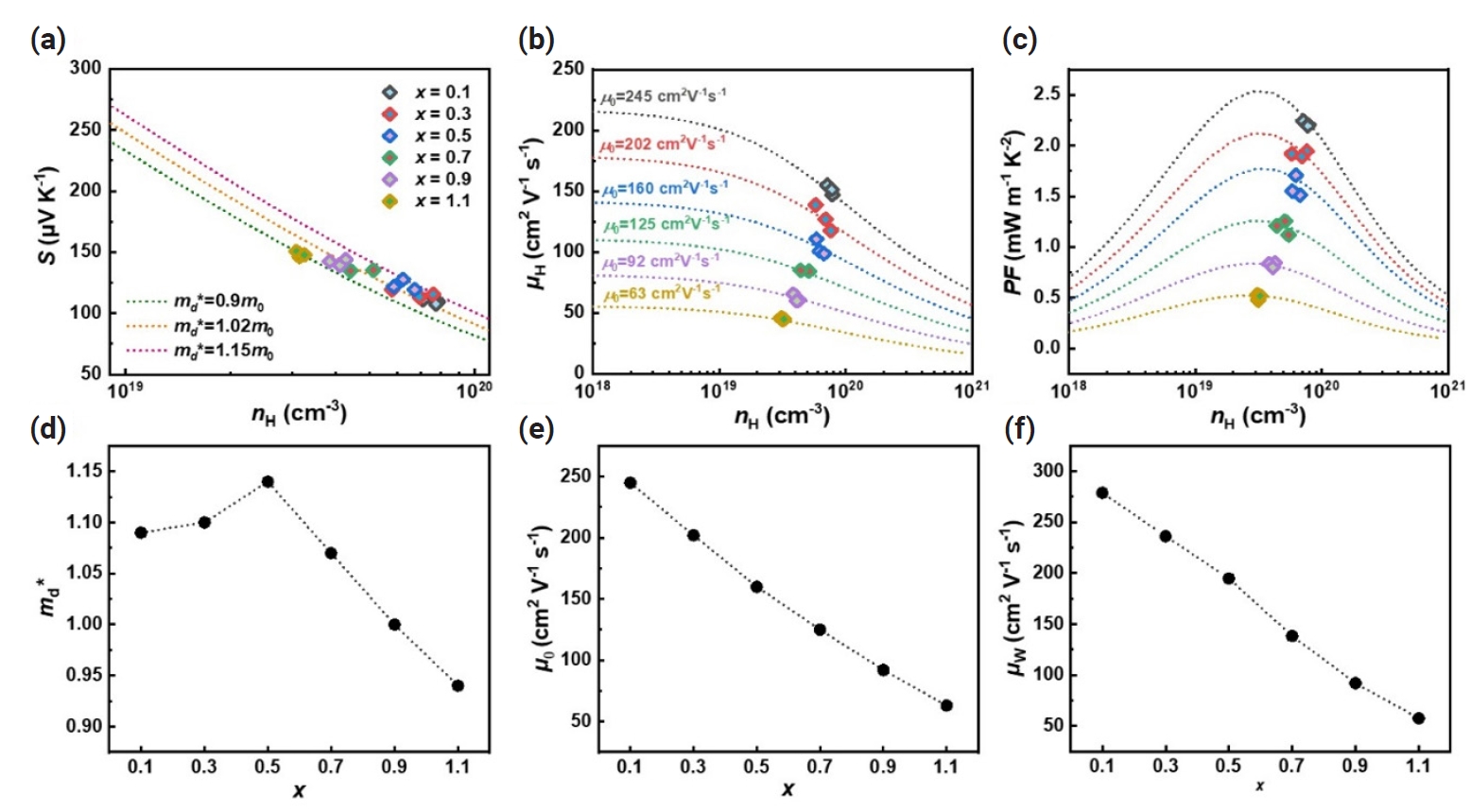
Hall carrier concentration (nH) dependent (a) Seebeck coefficient (S), (b) Hall carrier mobility (μH), and (c) power factor (S2σ) of Bi2-xSbxTe3 (x = 0.1 – 1.1). (d) density-of-state effective mass (md*), (e) nondegenerate mobility (μ0), and weighted mobility (μw) as a function of x. The single parabolic band (SPB) modeling was conducted using experimentally measured values at room temperature.
where kB, e, Fj, η, and h are the Boltzmann constant, elemental electric charge, Fermi integral of order j, reduced Fermi energy, and Planck’s constant, respectively. The estimated values of md* of Bi2-xSbxTe3 (x = 0.1 – 1.1) range from ~0.9 for x = 1.1 to 1.15 m0 for x = 0.5 where m0 is the free electron mass (Fig. 3d). These variations in md* could be attributed to changes in band curvature that occur with compositional changes [34]. In addition, we obtained nH dependent μH (nH-μH) curves for a given μ0 using the following equations (Fig. 3b),
where Cl, mb* and Edef are the elastic constant, single band effective mass and deformation potential, respectively. The estimated μ0 values range from ~245 cm2 V-1 s-1 for x = 0.1 to 63 cm2 V-1 s-1 for x = 1.1, showing a decreasing trend with increasing x (Fig. 3e). Given that μ0 is inversely proportional to the mb*, it is reasonable that μ0 decreased with increasing x up to x = 0.5. However, for x > 0.5, the md* and μ0 decreased simultaneously, which might be a result of the intensified carrier scattering induced by enhanced atomic disorder [35]. Fig. 3c shows the nH-dependent power factor (nH-S2σ) curve predicted by the SPB model, which agrees well with experimentally obtained S2σ at all compositions. Also, it is noted that with smaller x, nH deviates more from the optimized value for maximum S2σ, suggesting that the electrical properties of Bi2-xSbxTe3 (x = 0.1 – 1.1) can be improved by adjusting nH. The weighted mobility (μw = μ0 (md*/m0)3/2) as a function of x was presented in Fig. 3(f), calculated using theoretically estimated md* and μ0 (Fig. 3f). The μw is the primary parameter for the electrical properties of a given material, as the maximum S2σ is in direct proportion to the μw [36]. As shown in Fig. 3(f), the calculated μw gradually decreases as x increases. Therefore, it is concluded that Bi2-xSbxTe3 exhibits the most favorable electronic transport properties at x = 0.1 thanks to the optimized balance between md* and μ0.
The temperature-dependent thermal properties of Bi2-xSbxTe3 (x = 0.1 – 1.1) are shown in Fig. 4. As x increases, the total thermal conductivity (κtot) decreases and the lowest κtot of ~1.02 W m-1 K-1 was observed at x = 1.1, which should be attributed to the reduced electronic contribution as we observed in Fig. 2(a). To further understand the changes in thermal properties, we calculated the lattice thermal conductivity (κL= κtot – κe) by subtracting the κe from the κtot. The κe was determined using the Wiedemann-Franz law (κe = L0σT), where L0 is the Lorenz number (in 10-8 W Ω K‒2). We determined L0 using the following equation proposed by Kim et al [37].
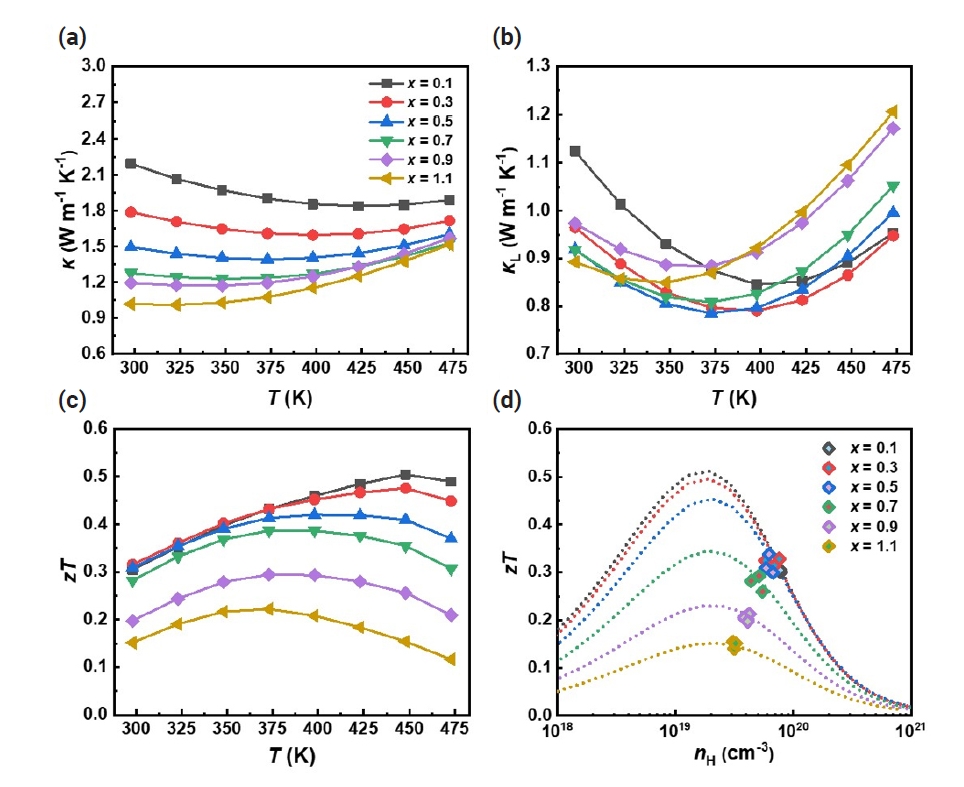
Temperature-dependent (a) total thermal conductivity (κtot), (b) lattice thermal conductivity (κL), and (c) figure of merit zT of Bi2-xSbxTe3 (x = 0.1 – 1.1). (d) Hall carrier concentration (nH) dependent zT (nH-zT) curves were estimated at room temperature using the single parabolic band (SPB) model.
The κL exhibited a decreasing trend as the Sb content increased with the lowest κL of ~0.9 W m-1 K-1 observed at x = 1.1 (Fig. 4b). The reduction of κL with increasing x could be attributed to the intensified point defect scattering as discussed in Fig. 3 and indicated by the following equation [29],
where x is the doping fraction, ΔM/M is the rate of change of relative atomic mass, adisorder and apure represent the lattice constants of disordered and pure alloys, and ε is an elasticity. It is also worth noting that κL decreased at elevated temperature with increasing x, suggesting the suppressed contribution of bipolar carriers due to increased nH [33].
The temperature-dependent figure of merit zT was evaluated from the measured σ, S, and κtot (Fig. 4c). The maximum zT value of ~0.5 was observed at 450 K for the x = 0.1 sample and the zT values decreased with increasing x within the measured temperature ranges. Furthermore, we obtained the nH-dependent zT (nH-zT) curve using the SPB model (Fig. 4d), and it was found that the zT values predicted based on the SPB model agree well with the experimentally evaluated ones. It is noteworthy that nH deviates more from the optimized value with lower Sb content (smaller x), indicating the need to optimize nH at lower x to further improve zT. According to the SPB model, zT can be improved by up to ~70% (e.g., from ~0.3 to ~0.5 for x = 0.1) by tuning nH at room temperature (Fig. 4d).
4. Conclusion
In this study, we investigated the thermoelectric properties and related band parameters of Bi2-xSbxTe3 (x = 0.1 – 1.1) using both experimental and theoretical approaches. Our findings, based on the estimation of weighted mobility (μw), revealed that the electronic transport properties are most favorable for x = 0.1, attributed to the balanced density-of-state effective mass (md*) and nondegenerate mobility (μ0). Additionally, at lower Sb content, the bipolar contribution to thermal conductivity is reduced at elevated temperatures. These synergetic effects led to the highest zT value of ~0.5 at 450 K in Bi1.9Sb0.1Te3. Furthermore, theoretical predictions by the single parabolic band (SPB) model suggest that the zT values can be further improved by up to ~70% through manipulating the carrier density. We believe that the current study not only contributes to the basic understanding of the n-type Bi2-xSbxTe3 but also expedites the development of high-performance n-type Bi2Te3-based thermoelectric materials.
Acknowledgements
This work was supported by KIMS' Principal R&D project (PNK9950) and the NRF' project (code no. 2021M3C1C3097540)of the Republc of Korea.