- [Korean]
- Fabrication of Metallic Tantalum Powder by Magnesium-gas Reduction of Tantalum Oxide
-
Dong-Won Lee
-
J Korean Powder Metall Inst. 2018;25(5):390-394. Published online October 1, 2018
-
DOI: https://doi.org/10.4150/KPMI.2018.25.5.390
-
-
858
View
-
14
Download
-
2
Citations
-
 Abstract Abstract
 PDF PDF
Metallic tantalum powder is manufactured by reducing tantalum oxide (Ta2O5) with magnesium gas at 1,073–1,223 K in a reactor under argon gas. The high thermodynamic stability of magnesium oxide makes the reduction reaction from tantalum oxide into tantalum powder possible. The microstructure after the reduction reaction has the form of a mixture of tantalum and magnesium oxide, and the latter could be entirely eliminated by dissolving in weak hydrochloric acid. The powder size in SEM microstructure for the tantalum powder increases after acid leaching in the range of 50–300 nm, and its internal crystallite sizes are observed to be 11.5 to 24.7 nm with increasing reduction temperatures. Moreover, the optimized reduction temperature is found to be 1,173 K as the minimum oxygen concentration is approximately 1.3 wt.%. -
Citations
Citations to this article as recorded by  - A review of tantalum resources and its production
Xue WEI, Long-gong XIA, Zhi-hong LIU, Le-ru ZHANG, Qi-hou LI
Transactions of Nonferrous Metals Society of China.2023; 33(10): 3132. CrossRef - Valuable metal recovery from waste tantalum capacitors via cryogenic crushing-alkaline calcination-leaching process
Longgong Xia, Xue Wei, Hongjun Wang, Fengchun Ye, Zhihong Liu
Journal of Materials Research and Technology.2022; 16: 1637. CrossRef
- [English]
- A Study on the Recovery of Li2CO3 from Cathode Active Material NCM(LiNiCoMnO2) of Spent Lithium Ion Batteries
-
Jei-Pil Wang, Jae-Jung Pyo, Se-Ho Ahn, Dong-Hyeon Choi, Byeong-Woo Lee, Dong-Won Lee
-
J Korean Powder Metall Inst. 2018;25(4):296-301. Published online August 1, 2018
-
DOI: https://doi.org/10.4150/KPMI.2018.25.4.296
-
-
3,496
View
-
76
Download
-
11
Citations
-
 Abstract Abstract
 PDF PDF
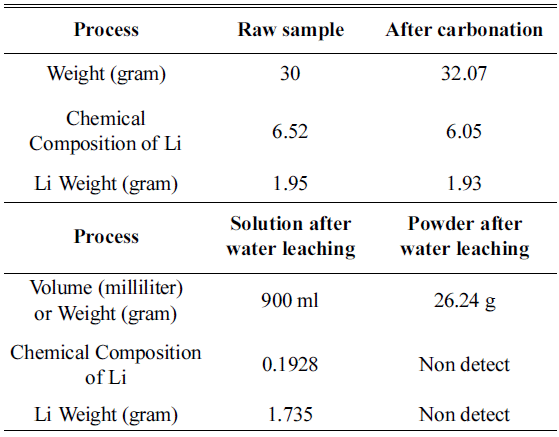
In this study, an experiment is performed to recover the Li in Li2CO3 phase from the cathode active material NMC (LiNiCoMnO2) in waste lithium ion batteries. Firstly, carbonation is performed to convert the LiNiO, LiCoO, and Li2MnO3 phases within the powder to Li2CO3 and NiO, CoO, and MnO. The carbonation for phase separation proceeds at a temperature range of 600°C~800°C in a CO2 gas (300 cc/min) atmosphere. At 600~700°C, Li2CO3 and NiO, CoO, and MnO are not completely separated, while Li and other metallic compounds remain. At 800 °C, we can confirm that LiNiO, LiCoO, and Li2MnO3 phases are separated into Li2CO3 and NiO, CoO, and MnO phases. After completing the phase separation, by using the solubility difference of Li2CO3 and NiO, CoO, and MnO, we set the ratio of solution (distilled water) to powder after carbonation as 30:1. Subsequently, water leaching is carried out. Then, the Li2CO3 within the solution melts and concentrates, while NiO, MnO, and CoO phases remain after filtering. Thus, Li2CO3 can be recovered. -
Citations
Citations to this article as recorded by  - Selective lithium Pre-Leaching from spent NMC black mass via pyrolysis (Carbothermal thermal Treatment) and controlled leaching
Amir Hossein Mohammad Zadeh, Spencer Cunnigham, Devon Gray, Sevan Bedrossian, Baian Almusned, Jeffrey Daniel Henderson, Gisele Azimi
Waste Management.2026; 212: 115339. CrossRef - Hydrogen Reduction of Black Mass Recovered from NCM-based Spent Lithium-ion Batteries
Jae-Ho Hwang, Sang-Yeop Lee, So-Yeong Lee, Ho-Sang Sohn
Resources Recycling.2025; 34(3): 34. CrossRef - Reduction Roasting of Cathode Materials of NCM Based Lithium-ion Batteries Using CH4(g)
Jae-Ho Hwang, Sang-Yeop Lee, Ho-Sang Sohn
Resources Recycling.2025; 34(4): 48. CrossRef - High‐Rate Rechargeable Li/SOCl2 Batteries Enabled by Cobalt Phthalocyanine Cathodic Catalysts
Yingxuan Song, Haibo Ouyang, Zhanwei Xu, Zeyang Zhang, Kang Li, Jianfeng Huang, Zhi Li, Tian Wang, Jun Yang
Small.2025;[Epub] CrossRef - Reduction Roasting of Black Mass Recovered from NCM-based Spent Lithium-ion Batteries Using CH4 Gas
Sang-Yeop Lee, Jae-Ho Hwang, Ho-Sang Sohn
Resources Recycling.2025; 34(5): 93. CrossRef - Metals Recovery from Spent Lithium-ion Batteries Cathode Via Hydrogen Reduction-water Leaching-carbothermic or Hydrogen Reduction Process
Tahereh Rostami, Behnam Khoshandam
Mining, Metallurgy & Exploration.2024; 41(3): 1485. CrossRef - Influence of Flow-Gas Composition on Reaction Products of Thermally Treated NMC Battery Black Mass
Christin Stallmeister, Bernd Friedrich
Metals.2023; 13(5): 923. CrossRef - Holistic Investigation of the Inert Thermal Treatment of Industrially Shredded NMC 622 Lithium-Ion Batteries and Its Influence on Selective Lithium Recovery by Water Leaching
Christin Stallmeister, Bernd Friedrich
Metals.2023; 13(12): 2000. CrossRef - Environmentally Friendly Recovery of Lithium from Lithium–Sulfur Batteries
Lilian Schwich, Bernd Friedrich
Metals.2022; 12(7): 1108. CrossRef - Early-Stage Recovery of Lithium from Tailored Thermal Conditioned Black Mass Part I: Mobilizing Lithium via Supercritical CO2-Carbonation
Lilian Schwich, Tom Schubert, Bernd Friedrich
Metals.2021; 11(2): 177. CrossRef - Exploring a green route for recycling spent lithium-ion batteries: Revealing and solving deep screening problem
Jiadong Yu, Quanyin Tan, Jinhui Li
Journal of Cleaner Production.2020; 255: 120269. CrossRef
- [Korean]
- Fabrication of TiC powder by carburization of TiH2 powder
-
Hun-Seok Lee, Hyang-Im Seo, Young-Seon Lee, Dong-Jun Lee, Jei-Pil Wang, Dong-Won Lee
-
J Korean Powder Metall Inst. 2017;24(1):29-33. Published online February 1, 2017
-
DOI: https://doi.org/10.4150/KPMI.2017.24.1.29
-
-
684
View
-
2
Download
-
1
Citations
-
 Abstract Abstract
 PDF PDF
Titanium carbide (TiC) powders are successfully synthesized by carburization of titanium hydride (TiH2) powders. The TiH2 powders with size lower than 45 μm (-325 Mesh) are optimally produced by the hydrogenation process, and are mixed with graphite powder by ball milling. The mixtures are then heat-treated in an Ar atmosphere at 800-1200oC for carburization to occur. It has been experimentally and thermodynamically determined that the dehydrogenation, “TiH2 = Ti + H2”, and carburization, “Ti + C = TiC”, occur simultaneously over the reaction temperature range. The unreacted graphite content (free carbon) in each product is precisely measured by acid dissolution and by the filtering method, and it is possible to conclude that the maximal carbon stoichiometry of TiC0.94 is accomplished at 1200°C. -
Citations
Citations to this article as recorded by  - Pre-treatments of initial materials for controlling synthesized TaC characteristics in the SHS process
Jae Jin Sim, Sang Hoon Choi, Ji Hwan Park, Il Kyu Park, Jae Hong Lim, Kyoung Tae Park
journal of Korean Powder Metallurgy Institute.2018; 25(3): 251. CrossRef
- [Korean]
- Synthesis of Nanostructured Ceria Powders for an Oxygen-sensor by Thermochemical Process
-
Dong-Won Lee, Joon-Hwan Choi, Tae-Soo Lim, Yong-Jin Kim
-
J Korean Powder Metall Inst. 2006;13(3):192-198.
-
DOI: https://doi.org/10.4150/KPMI.2006.13.3.192
-
-
 Abstract Abstract
 PDF PDF
- The nanostructured cerium oxide powders were synthesized by spray thermal decomposition process for the use as the raw materials of resistive oxygen sensor. The synthesis routes consisted of 1) spray drying of water based organic solution made from cerium nitrate hydrate (Ce(NO_3)_36H_2O) and 2) heat treatment of spray dried precursor powders at 400°C in air atmosphere to remove the volatile components and identically to oxidize the cerium component. The produced powders have shown the loose structure agglomerated with extremely fine cerium oxide particles with about 15 nm and very high specific surface area (110m2/g). The oxygen sensitivity, n (LogproptoLog (P_O2/Po)-n and the response time, t_90 measured at 600°C in the sample sintered at 1000°C, were about 0.25 and 3 seconds, respectively, which had much higher performances than those known in micron or 100sim200nm sized sensors.
- [Korean]
- A Study on Oxidation-Resistance of Iron Nanoparticles Synthesized by Chemical Vapor Condensation Process
-
Dong-Won Lee, Ji-Hun Yu, Jeoung-Hyun Bae, Tae-Suk Jang, Byoung-Kee Kim
-
J Korean Powder Metall Inst. 2005;12(3):225-230.
-
DOI: https://doi.org/10.4150/KPMI.2005.12.3.225
-
-
 Abstract Abstract
 PDF PDF
- In order to prevent the oxide formation on the surface of nano-size iron particles and thereby to improve the oxidation resistance, iron nanoparticles synthesized by a chemical vapor condensation method were directly soaked in hexadecanethiol solution to coat them with a polymer layer. Oxygen content in the polymer-coated iron nanoparticles was significantly lower than that in air-passivated particles possessing iron-core/oxide-shell structure. Accordingly, oxidation resistance of the polymer-coated particles at an elevated temperature below 130°C in air was 10~40 times higher than that of the air- passivated particles.
|