- [Korean]
- Synthesis and Morphology Control of Needle Type 513 MHSH and Mg(OH)2 from Dolomite
-
Jiyeon Kim, HyunSeung Shim, Seong-Ju Hwang, YooJin Kim
-
J Powder Mater. 2025;32(5):399-405. Published online October 31, 2025
-
DOI: https://doi.org/10.4150/jpm.2025.00227
-
-
 Abstract Abstract
 PDF PDF
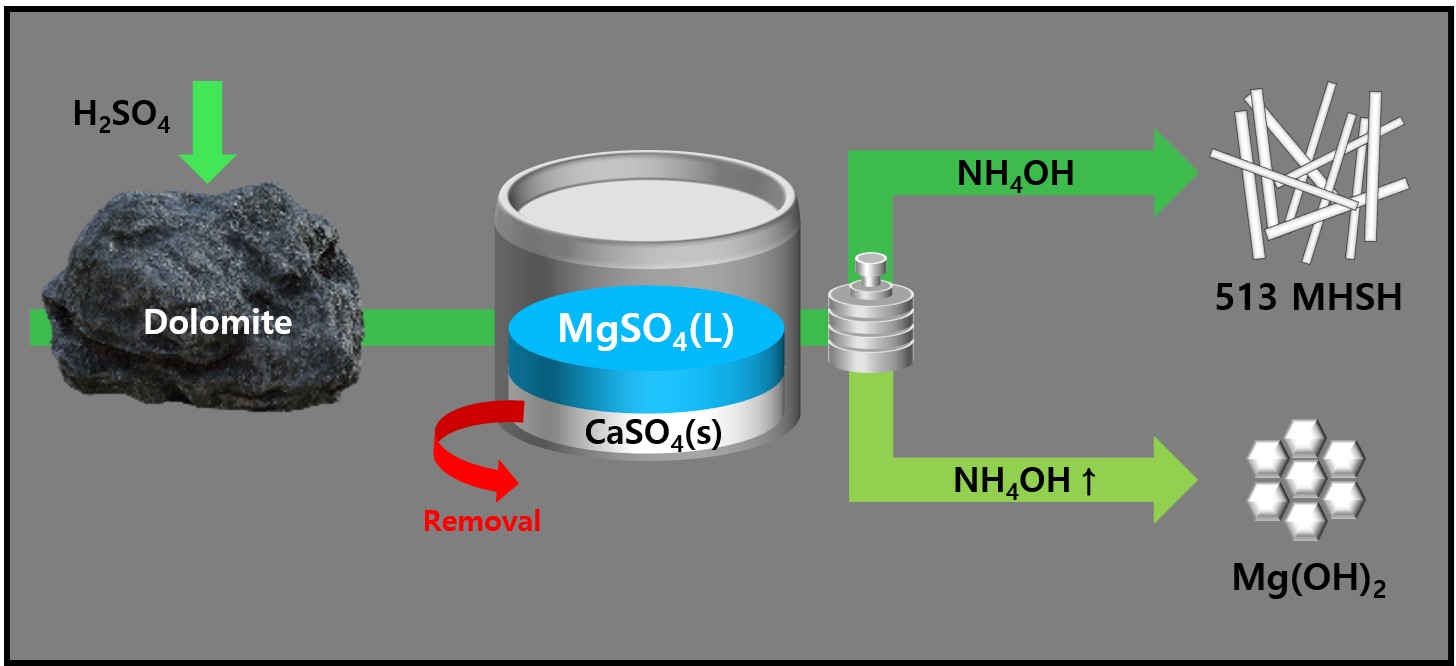
- 513 magnesium hydroxide sulfate hydrate (MHSH) and Mg(OH)₂ were synthesized by controlling the pH and concentration using a domestic resource, dolomite (CaMg(CO3)2), as the raw material. The MgSO₄ was extracted by treating dolomite with sulfuric acid under various conditions. Hexagonal plate-shaped Mg(OH)₂ and needle-like 513 MHSH were synthesized under the hydrothermal condition. The morphology of the synthesized materials was controlled by adjusting the pH (SO42-/OH- ratio) and hydrothermal reaction time. As the pH of the solution increased, the formation of plate-like structures became dominant, whereas lower pH values (higher SO42- concentration) led to needle-like forms. The results of the 513 MHSH, which was synthesized using reagents and sea bittern, are consistent with the synthesis conditions, and we observed changes in the length and aspect ratio of the needle-shaped structure in response to adjusting the hydrothermal reaction time.
- [Korean]
- Extraction of MgSO4 from dolomite and synthesis of Mg(OH)2 in Bittern
-
HyunSeung Shim, Jiyeon Kim, Areum Choi, Nuri Oh, YooJin Kim
-
J Powder Mater. 2025;32(2):122-130. Published online April 30, 2025
-
DOI: https://doi.org/10.4150/jpm.2025.00073
-
-
946
View
-
33
Download
-
1
Citations
-
 Abstract Abstract
 PDF PDF
- We synthesized magnesium hydroxide using bittern and dolomite, which are domestic resources. In Bittern, there is a high concentration of Mg2+ ions, but the impurity Ca2+ ion content is also significant, requiring a purification process to remove it. There are two main methods for this purification. Firstly, there is a separation method that utilizes the difference in solubility between Mg2+ ions and Ca2+ ions by using sulfuric acid on dolomite. Adding MgSO4 solution from dolomite to Bittern removes Ca2+ ions as CaSO4. This process simultaneously purifies Ca impurities and increases the Mg/Ca ratio by adding extra Mg2+ ions. In this study, purified bittern was obtained by using dolomite and sulfuric acid to extract MgSO4, which was then used to purify Ca2+ ions. High-purity Mg(OH)2 was synthesized by optimizing the NaOH and NH4OH ratio as an alkaline precipitant.
-
Citations
Citations to this article as recorded by  - Synthesis and Morphology Control of Needle Type 513 MHSH and Mg(OH)2 from Dolomite
Jiyeon Kim, HyunSeung Shim, Seong-Ju Hwang, YooJin Kim
Journal of Powder Materials.2025; 32(5): 399. CrossRef
|