Search
- Page Path
- HOME > Search
- [Korean]
- Recent Development in Fabrication and Control of Layered-Double Hydroxide Nanostructures
- Chan-Woo Jeon, Il-Kyu Park
- J Korean Powder Metall Inst. 2018;25(6):514-522. Published online December 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.6.514
- 1,553 View
- 23 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
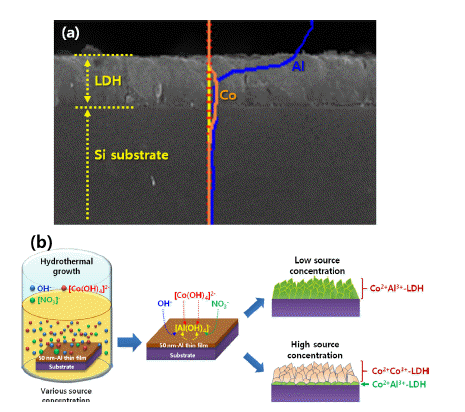
PDF Layered-double hydroxide (LDH)-based nanostructures offer the two-fold advantage of being active catalysts with incredibly large specific surface areas. As such, they have been studied extensively over the last decade and applied in roles as diverse as light source, catalyst, energy storage mechanism, absorber, and anion exchanger. They exhibit a unique lamellar structure consisting of a wide variety of combinations of metal cations and various anions, which determine their physical and chemical performances, and make them a popular research topic. Many reviewed papers deal with these unique properties, synthetic methods, and applications. Most of them, however, are focused on the form-factor of nanopowder, as well as on the control of morphologies via one-step synthetic methods. LDH nanostructures need to be easy to control and fabricate on rigid substrates such as metals, semiconductors, oxides, and insulators, to facilitate more viable applications of these nanostructures to various solid-state devices. In this review, we explore ways to grow and control the various LDH nanostructures on rigid substrates.
-
Citations
Citations to this article as recorded by- Review of Domestic Research Trends on Layered Double Hydroxide (LDH) Materials: Based on Research Articles in Korean Citation Index (KCI)
Seon Yong Lee, YoungJae Kim, Young Jae Lee
Economic and Environmental Geology.2023; 56(1): 23. CrossRef
- Review of Domestic Research Trends on Layered Double Hydroxide (LDH) Materials: Based on Research Articles in Korean Citation Index (KCI)
- [Korean]
- Morphology Control of ZnO Nanostructures by Surfactants During Hydrothermal Growth
- Il-Kyu Park
- J Korean Powder Metall Inst. 2016;23(4):270-275. Published online August 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.4.270
- 774 View
- 1 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
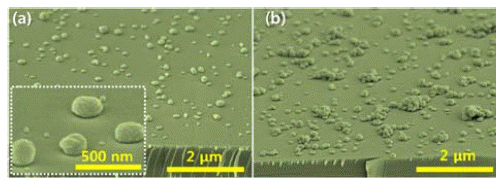
PDF We report on an all-solution-processed hydrothermal method to control the morphology of ZnO nanostructures on Si substrates from three-dimensional hemispherical structures to two-dimensional thin film layers, by controlling the seed layer and the molar contents of surfactants during their primary growth. The size and the density of the seed layer, which is composed of ZnO nanodots, change with variation in the solute concentration. The ZnO nanodots act as heterogeneous nucleation sites for the main ZnO nanostructures. When the seed layer concentration is increased, the ZnO nanostructures change from a hemispherical shape to a thin film structure, formed by densely packed ZnO hemispheres. In addition, the morphology of the ZnO layer is systematically controlled by using trisodium citrate, which acts as a surfactant to enhance the lateral growth of ZnO crystals rather than a preferential one-dimensional growth along the c-direction. X-ray diffraction and energy dispersive X-ray spectroscopy results reveal that the ZnO structure is wurtzite and did not incorporate any impurities from the surfactants used in this study.
-
Citations
Citations to this article as recorded by- La-doped p-type ZnO nanowire with enhanced piezoelectric performance for flexible nanogenerators
Leeseung Kang, HyeLan An, Ji Young Park, Myung Hwan Hong, Sahn Nahm, Chan Gi Lee
Applied Surface Science.2019; 475: 969. CrossRef
- La-doped p-type ZnO nanowire with enhanced piezoelectric performance for flexible nanogenerators
TOP
 KPMI
KPMI


 First
First Prev
Prev


