Search
- Page Path
- HOME > Search
- [Korean]
- Study on Surface-defect Passivation of InP System Quantum Dots by Photochemical Method
- Doyeon Kim, Hyun-Su Park, Hye Mi Cho, Bum-Sung Kim, Woo-Byoung Kim
- J Korean Powder Metall Inst. 2017;24(6):489-493. Published online December 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.6.489
- 1,602 View
- 12 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
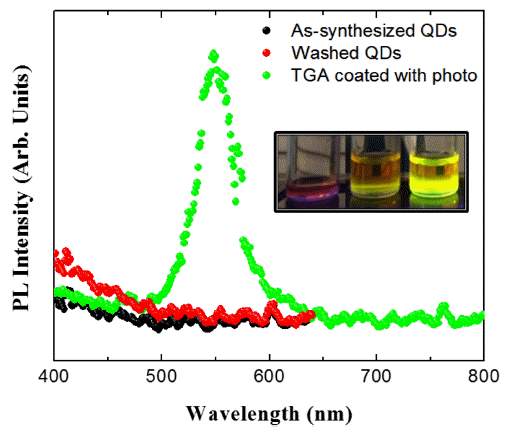
PDF In this study, the surface passivation process for InP-based quantum dots (QDs) is investigated. Surface coating is performed with poly(methylmethacrylate) (PMMA) and thioglycolic acid. The quantum yield (QY) of a PMMA-coated sample slightly increases by approximately 1.3% relative to that of the as-synthesized InP/ZnS QDs. The QYs of the uncoated and PMMA-coated samples drastically decrease after 16 days because of the high defect state density of the InP-based QDs. PMMA does not have a significant effect on the defect passivation. Thioglycolic acid is investigated in this study for the effective surface passivation of InP-based QDs. Surface passivation with thioglycolic acid is more effective than that with the PMMA coating, and the QY increases from 1.7% to 11.3%. ZnS formed on the surface of the InP QDs and S in thioglycolic acid show strong bonding property. Additionally, the QY is further increased up to 21.0% by the photochemical reaction. Electron–hole pairs are formed by light irradiation and lead to strong bonding between the inorganic and thioglycolic acid sulfur. The surface of the InP core QDs, which does not emit light, is passivated by the irradiated light and emits green light after the photochemical reaction.
-
Citations
Citations to this article as recorded by- Poly(methylmethacrylate) coating on quantum dot surfaces via photo-chemical reaction for defect passivation
Doyeon Kim, So-Yeong Joo, Chan Gi Lee, Bum-Sung Kim, Woo-Byoung Kim
Journal of Photochemistry and Photobiology A: Chemistry.2019; 376: 206. CrossRef
- Poly(methylmethacrylate) coating on quantum dot surfaces via photo-chemical reaction for defect passivation
- [Korean]
- Optical Characteristics of CdSe/ZnS Quantum Dot with Precursor Flow Rate Synthesized by using Microreactor
- Ji Young Park, Da-Woon Jeong, Won Ju, Han Wook Seo, Yong-Ho Choa, Bum Sung Kim
- J Korean Powder Metall Inst. 2016;23(2):91-94. Published online April 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.2.91
- 1,279 View
- 6 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
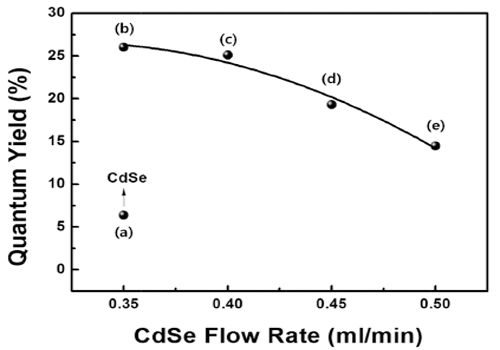
PDF High-quality colloidal CdSe/ZnS (core/shell) is synthesized using a continuous microreactor. The particle size of the synthesized quantum dots (QDs) is a function of the precursor flow rate; as the precursor flow rate increases, the size of the QDs decreases and the band gap energy increases. The photoluminescence properties are found to depend strongly on the flow rate of the CdSe precursor owing to the change in the core size. In addition, a gradual shift in the maximum luminescent wave (λmax) to shorter wavelengths (blue shift) is found owing to the decrease in the QD size in accordance with the quantum confinement effect. The ZnS shell decreases the surface defect concentration of CdSe. It also lowers the thermal energy dissipation by increasing the concentration of recombination. Thus, a relatively high emission and quantum yield occur because of an increase in the optical energy emitted at equal concentration. In addition, the maximum quantum yield is derived for process conditions of 0.35 ml/min and is related to the optimum thickness of the shell material.
-
Citations
Citations to this article as recorded by- Quantum materials made in microfluidics - critical review and perspective
M. Wojnicki, V. Hessel
Chemical Engineering Journal.2022; 438: 135616. CrossRef - Poly(methylmethacrylate) coating on quantum dot surfaces via photo-chemical reaction for defect passivation
Doyeon Kim, So-Yeong Joo, Chan Gi Lee, Bum-Sung Kim, Woo-Byoung Kim
Journal of Photochemistry and Photobiology A: Chemistry.2019; 376: 206. CrossRef - Multimodal luminescence properties of surface-treated ZnSe quantum dots by Eu
Ji Young Park, Da-Woon Jeong, Kyoung-Mook Lim, Yong-Ho Choa, Woo-Byoung Kim, Bum Sung Kim
Applied Surface Science.2017; 415: 8. CrossRef
- Quantum materials made in microfluidics - critical review and perspective
TOP
 KPMI
KPMI


 First
First Prev
Prev


