Search
- Page Path
- HOME > Search
- [Korean]
- Photoluminescence Enhancement of Y2O3:Eu3+ Red Phosphor Prepared by Spray Pyrolysis using Aliovalent Cation Substitution and Organic Additives
- Byeong Ho Min, Kyeong Youl Jung
- J Korean Powder Metall Inst. 2020;27(2):146-153. Published online April 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.2.146
- 660 View
- 4 Download
-
 Abstract
Abstract
 PDF
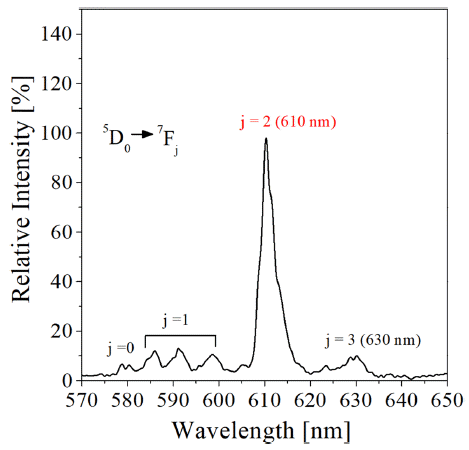
PDF The co-doping effect of aliovalent metal ions such as Mg2+, Ca2+, Sr2+, Ba2+, and Zn2+ on the photoluminescence of the Y2O3:Eu3+ red phosphor, prepared by spray pyrolysis, is analyzed. Mg2+ metal doping is found to be helpful for enhancing the luminescence of Y2O3:Eu3+. When comparing the luminescence intensity at the optimum doping level of each Mg2+ ion, the emission enhancement shows the order of Zn2+ ≈ Ba2+ > Ca2+ > Sr3+> Mg2+. The highest emission occurs when doping approximately 1.3% Zn2+, which is approximately 127% of the luminescence intensity of pure Y2O3:Eu3+. The highest emission was about 127% of the luminescence intensity of pure Y2O3:Eu3+ when doping about 1.3% Zn2+. It is determined that the reason (Y, M)2O3:Eu3+ has improved luminescence compared to that of Y2O3:Eu3+ is because the crystallinity of the matrix is improved and the non-luminous defects are reduced, even though local lattice strain is formed by the doping of aliovalent metal. Further improvement of the luminescence is achieved while reducing the particle size by using Li2CO3 as a flux with organic additives.
- [Korean]
- Preparation of Nanosized Gd2O3:Eu3+ Red Phosphor Coated on Mica Flake and Its Luminescent Property
- Se-Min Ban, Jeong Min Park, Kyeong Youl Jung, Byung-Ki Choi, Kwang-Jung Kang, Myung Chang Kang, Dae-Sung Kim
- J Korean Powder Metall Inst. 2017;24(6):457-463. Published online December 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.6.457

- 925 View
- 4 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Nanosized Gd2O3:Eu3+ red phosphor is prepared using a template method from metal salt impregnated into a crystalline cellulose and is dispersed using a bead mill wet process. The driving force of the surface coating between Gd2O3:Eu3+ and mica is induced by the Coulomb force. The red phosphor nanosol is effectively coated on mica flakes by the electrostatic interaction between positively charged Gd2O3:Eu3+ and negatively charged mica above pH 6. To prepare Gd2O3:Eu3+-coated mica (Gd2O3:Eu/mica), the coating conditions are optimized, including the stirring temperature, pH, calcination temperature, and coating amount (wt%) of Gd2O3:Eu3+. In spite of the low luminescence of the Gd2O3:Eu/mica, the luminescent property is recovered after calcination above 600°C and is enhanced by increasing the Gd2O3:Eu3+ coating amount. The Gd2O3:Eu/mica is characterized using X-ray diffraction, field emission scanning electron microscopy, zeta potential measurements, and fluorescence spectrometer analysis.
-
Citations
Citations to this article as recorded by- Optimization of dispersed LaPO4:Tb nanosol and their photoluminescence properties
Mahboob Ullah, Se-Min Ban, Dae-Sung Kim
Optical Materials.2019; 97: 109366. CrossRef
- Optimization of dispersed LaPO4:Tb nanosol and their photoluminescence properties
TOP
 KPMI
KPMI


 First
First Prev
Prev


