Articles
- Page Path
- HOME > J Korean Powder Metall Inst > Volume 21(1); 2014 > Article
-
ARTICLE
- Advanced PM Processes for Medical Technologies
- Frank Petzoldt*, Vera Friederici, Philipp Imgrund, Claus Aumund-Kopp
-
Journal of Korean Powder Metallurgy Institute 2014;21(1):1-6.
DOI: https://doi.org/10.4150/KPMI.2014.21.1.1
Published online: January 31, 2014
- *Corresponding Author : Frank Petzoldt, TEL: +49-421-2246-134, FAX: +49-421-2246-300, E-mail: frank.petzoldt@ifam.fraunhofer.de
• Received: December 17, 2013 • Accepted: January 17, 2014
© Korean Powder Metallurgy Institute
- 950 Views
- 5 Download
- 2 Crossref
Abstract
- Medical technologies are gaining in importance because of scientific and technical progress in medicine and the increasing average lifetime of people. This has opened up a huge market for medical devices, where complex-shaped metallic parts made from biocompatible materials are in great demand. Today many of these components are already being manufactured by powder metallurgy technologies. This includes mass production of standard products and also customized components. In this paper some aspects related to metal injection molding of Ti and its alloys as well as modifications of microstructure and surface finish were discussed. The process chain of additive manufacturing (AM) was described and the current state of the art of AM processes like Selective Laser Melting and electron beam melting for medical applications was presented.
- Due to recent advances in the medical science as well as the continuous increase in the average human lifespan, there is a growing demand for medical technologies that help people fight illness, disabilities and age-related degeneration and thus gain better quality of life. This has opened up a growing market for all kinds of medical devices, where complex-shaped metallic components that meet the strict requirements and quality standards in medicine are of vital interest. For the entire powder metallurgy industry, this market creates many interesting business opportunities, beginning with specially tailored powders or feedstocks, production process adaptations for increased quality and developing new surface finishing treatments.
- Looking at the market demands in medical technology, one can recognize two clearly defined trends which can both be served by modern powder-based production processes: Large-scale series manufacturing and product customization. Mass production of complex metallic components can be achieved with the well-established production process MIM, if all the mandatory requirements and specifications for medical components are met. The choice of material is defined by the type of product and its requirements on biocompatibility, mechanical and corrosion properties. The longer the product is in contact with human tissue, the higher its biocompatibility rating needs to be. For example a long-term hip implant needs the highest degree of biocompatibility - preferably even a bioactive surface - whereas the needle of a single-use syringe, in use for a few seconds, just needs to be basically bioinert.
- Also, there are numerous standards on materials and products (e.g. chemical composition and properties) in medical technology that need to be met. For example, the international ISO standard 5832-3 specifies the material characteristics and testing methods of wrought Ti-6Al-4V for use in the manufacturing of surgical implants. The characteristics of wrought Ti-6Al-4V for implant applications are also specified in the American ASTM standard F136-13, whereas metal injection molded Ti-6Al-4V for implant applications is covered by ASTM F2885-11. Companies that produce medical products need to comply with ISO 13485, which defines the standard procedures for a comprehensive quality management system for the design, production and marketing of medical products. Being certified according to ISO 13485 usually serves as a first step for achieving compliance with several European regulatory requirements on medical products, implants and diagnostics and ultimately the permission to sell medical products in the European Union.
Introduction
- Metal Injection Molding (MIM) is nowadays a very reliable production process for complex-shaped serial parts that have found widespread use in all industrial markets. A very well-established mass production process in medical technology is the injection molding of small components for orthodontics, like e.g. brackets and buccal tubes [1]. These are usually made from medicalgrade, nickel-free stainless steel, which today counts among the standard materials for the metal injection molding process and is commercially available as powder and ready-to-use feedstock. Still, for this particular market segment, there are many other materials for which a reliable mass-production process like MIM would be highly desirable, but for each new material and application, the process must be adapted and fine-tuned individually. Due to its biocompatibility, low specific weight and excellent mechanical properties, titanium and its alloys are very interesting for the use in medical applications. The availability of fine powders with a spherical particle shape and low impurity levels is a prerequisite for the success of titanium MIM. The price of these powders still restricts the application of titanium and its alloys to small components or high-end specialty products. Furthermore, it is important to guarantee a reproducible process chain focusing on impurity control, as well as titanium grades that can be processed in series production equipment.
- One additional advantage of titanium is that it encourages in-vivo rapid bone formation at the implant surface. Therefore it is seen as the optimal material for bone implants. However, pure titanium is very reactive and picks up light elements (oxygen, nitrogen, carbon) rapidly, which poses a real challenge when debinding and sintering titanium parts. In particular, high levels of oxygen are often found in titanium powder sources. Also, carbon can be involuntarily but easily introduced due to insufficient binder removal prior to sintering or to damaging reactions between the decomposing binder, the debinding atmosphere, and the metal phase. Even at low concentrations, these impurities can severely impair the mechanical properties of the finished part [2]. In order to minimize contamination of the metal injection molded titanium parts, the suitability of different wax/polymer based binders was tested.
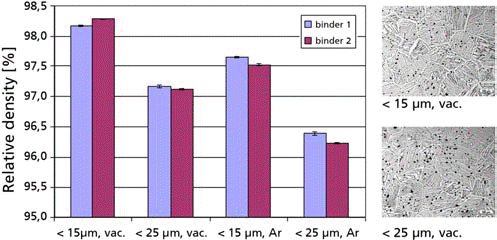
- Fig. 1 shows that the highest density is achieved by using powders with a particle size < 15 μm and vacuum sintering. The influence of the chosen binder is minor compared to the influence of the sintering atmosphere. A comparison of MIM Ti-6Al-4V with the standard for Ti grade 5 shows that the impurity level and strength are almost met, but the elongation is lower than expected.
- Based on these promising results, a MIM process chain for the series production of titanium interference screws for cruciate ligament fixation has been developed. The entire screw geometry including thread and hexagonal socket can be molded and sintered without further finishing treatment, and the material properties of the finished screw meet all medical requirements [3].
- There are other interesting Ti alloys for the injection molding of medical components. So-called shape memory alloys possess the unique feature of changing into a predefined shape at a certain temperature. For surgical implants like e.g. stents, this means that by cooling the component during surgery to below 37°C the shape can be completely different (e.g. smaller, therefore easier to insert) from the shape that the part will assume in the body where it reaches the transition temperature. To broaden potential applications of MIM components the processing of pre-alloyed NiTi powder with a phase transformation temperature of 37°C by MIM has been evaluated. The results of the injection molding and sintering experiments are promising, the test parts have reached an average density of 96 to 98% after sintering, and the NiTi phase is dominant.
Metal Injection Molding of Titanium and Titanium Alloys
- To further improve integration behavior in implants, the interaction between implant and body cells can be managed by introducing bioactive surfaces. This is usually done either by finishing operations, such as etching or sandblasting, or by the application of bioactive coatings. A very interesting aspect of MIM production is the possibility to equip implants with bioactive surfaces directly during the injection molding process by molding microsized patterns on the surface of the part, which influence the surface roughness of the part and, depending on their shape and distance, either encourage or inhibit cell growth [4, 5].
- In a series of studies with different metallic and nonmetallic implant materials, it was found that regular micro patterns on the surfaces can significantly enhance the integration of implants into the body. For example, hexagonally arranged protruding hemispheres with a diameter between 5 μm and 50 μm showed the most promising cell migration and differentiation results on polymer substrates [6]. In the example presented here, small test specimens of stainless steel 316L with a microstructured surface of hemispheres with 50 μm diameter and 20 μm interspacing as shown in Fig. 2 were produced by microinjection molding (μ-MIM).
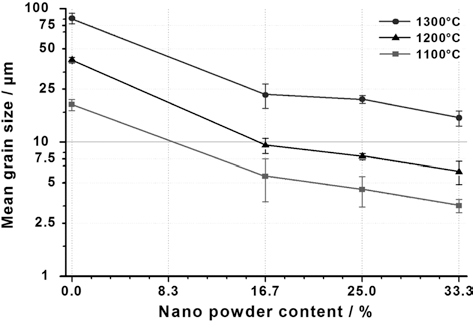
- The dimensional accuracy of the test specimens and their excellent reproducibility were achieved by adapting the injection molding process for micro-sized applications. A special powder mixture containing a master alloy powder Cr55Ni38Mo7, ultra fine Fe particles (d50 =1.4 μm) and a percentage of nano-sized Fe powder particles (d50 = 17 nm) was used as base for the feedstock. Due to the nano powder content, special measures had to be taken for feedstock preparation. Binder content had to be increased and the entire kneading process had to be done under inert gas atmosphere to protect the nanoparticles from oxidation. In order to achieve complete form filling and good green part stability, the feedstock and molding temperature had to be increased and injection pressure had to be lowered. An additional welcome effect of the powder mix modification was the improvement of the microstructure of all test parts. Fig. 3 shows clearly that an increasing content of nanopowder particles and the correct adjustment of sintering temperatures lead to an increasing grain refinement in the microstructure, endowing the microstructured surface with a secondary, nanosized substructure which adds a further positive influence to surface conditions that are already apt for good cell adhesion. Fine substructures, especially in the submicron range, have been shown to support osteoblast attachment and differentiation [7]. First results related to immunohystochemistry that were performed by EMPA Materials Science and Technology, Switzerland, indicated significant bioactive stimulation resulting from the presence of the hemispheres. Furthermore polymerase chain reaction (PCR) experiments revealed an enhanced osteogenic differentiation in cells seeded on the structured surfaces compared to cell culture control surfaces.
- The micrographs in Fig. 4 show the influence of a 33% Fe nanoparticles addition on the formation of microstructure.
Improved Biocompatibility by Surface Modification
- Additive manufacturing (AM) is a layer by layer production technology to produce complex shaped components as customized components or in small series applications. Today there are many different machine concepts in the market following the principle of producing parts directly from CAD data. It is possible to manufacture e.g. dental or bone replacement implants that are fully customized in size and shape for individual patients. Selective Laser Melting (SLM, also known as Laser Sintering) is the most mature metal-based AM process. Nowadays, parts produced with SLM systems have achieved a level of quality that makes them competitive to conventionally produced parts with regard to material properties like density and strength. Also geometrical accuracy has reached values of 20 μm which is sufficient level for many applications. Today surface quality in the as-sintered state has also improved to Ra values of 10 μm. Allowances for necessary post processing can thus be minimized and planned accordingly.
- Especially in the field of dental restorations, the SLM process is continuously taking over part of the market share for producing conventional dental replacement. It allows to manufacture individualized dental abutments, crown and bridge frames [8]. These dental restorations are produced by laser melting from special cobalt-chrome alloys based on the CAD data created from the patient’s dental imprint. Some hundred individual dental parts are produced on the same building platform within one production cycle. Although the reliability of the production is already very good a tensile test specimen is built on each building platform for quality control. After mechanical separation from the building platform, the parts are being subjected to a thorough inspection for size before being shipped to the dental technician for finishing treatment and veneering. Delivery of the metallic components is guaranteed within 48 hours, which makes SLM a very competitive technology.
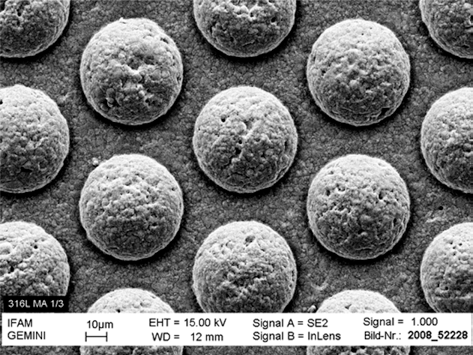
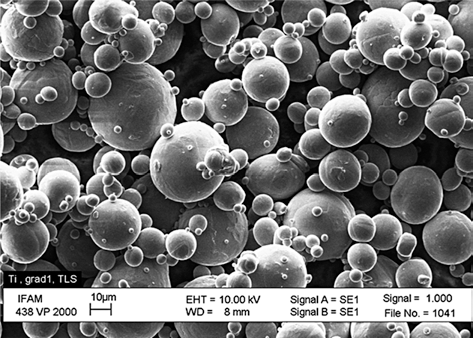
- The powders used for SLM have to fulfill certain specifications, the most important being a spherical shape within a narrow particle distribution range. No binders or additives are needed in the SLM construction process, the powder needs to be completely humidity free. Fig. 5 shows a gas atomized Ti powder with a d50 of 20 μm. The particle size defines the minimum layer thickness. In case of Ti powders, the SLM process also needs to be carried out inert gas (Ar) atmosphere to minimize potential reactions with oxygen or nitrogen.
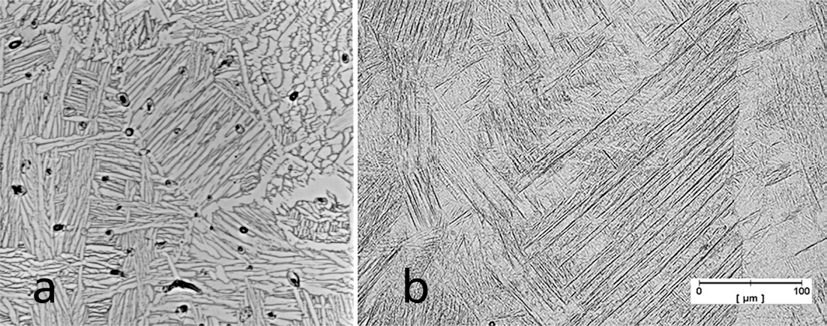
- A comparison of the microstructure of laser sintered Ti-6Al-4V to the injection molded material in Fig. 6 shows differences resulting from the rapid solidification during SLM. Fig. 6(b) shows a dense structure (isolated pores) and dendritic/martensitic microstructure with preferred direction. The grain size is larger than the layer thickness.
- In Table 2 a comparison of Ti-6Al-4V properties produced by SLM and MIM is shown. The impurity level of powder especially the oxygen content has to be as low as possible and must be controlled during processing by choosing an appropriate sintering atmosphere for MIM and also in the processing chamber of the SLM machine. If the laser melting process is carried out correctly, the finished parts have nearly full density, excellent mechanical properties and meet ASTM standards F136 and F1472 and thus can be qualified for the use in medical applications.
- The possibility to combine different material properties or structural elements is a unique feature of AM processes. Fig. 7 presents the possible material combinations of porous and dense areas of a part in detail. The building layers of the manufacturing process can be clearly observed. A dense shell area combined with a porous core area could be used for filtering or heat exchange purposes as well as for lightweight designs. The porous shell – dense core approach (Fig. 7b) is useful when the above mentioned osteosynthesis or other biological activities have to be fostered. A possible product application for this manufacturing approach would be e.g. a femur implant – directly integrating a dense main body offering the necessary mechanical stability, which in turn is surrounded by a porous shell structure providing ideal conditions for a good osteosynthesis [9].
- A second outstanding feature of the SLM technology is the possibility to manufacture complex thin walled structures or parts. With standard process parameters and conditions, dense walls with thicknesses of 0.2 mm can be produced. If even smaller thicknesses in combination with complex designs are needed, the SLM process can be combined with electro polishing as finishing treatment.
- Another AM process for metallic components that conform to the ASTM standards for surgical implants is electron beam melting (EBM). Instead of a laser beam, the metal powder is melted by an electron beam under vacuum conditions, thus allowing the production of fully dense materials, e.g. from Ti alloys. As a result of reduced residual stress, Ti-6Al-V4 parts manufactured by EBM exhibit greater ductility when compared to laser melted material. The powders used in EMB are coarser (about 100μm) than those for SLM, resulting in a much faster building rate and therefore making the process economic for larger components. Due to the coarser powder the achievable surface roughness for EBM components is higher than that produced by SLM, which can even be beneficial for or some components like e.g. acetabular cups for hip implants, which are inserted into a freshly drilled bone cavity, where rough surfaces can help to minimize implant movement and encourage bone ingrowth. Specially tailored EBM surface structures have been developed and tested for maximum effectiveness in that regard [10].
Individual Medical Components by Additive Manufacturing
- MIM technology has already proven its potential as a mature mass production process for metallic components in medical applications. Today it is possible to manufacture serial products fulfilling all regulatory requirements and standards. Especially Ti and its alloys require advanced processes to achieve competitive material properties. There are further chances for MIM by introducing special powder mixes including nanosized powders to design microstructures and optimized surfaces for an enhanced cellgrowth interaction of components implanted in the human body. Special bio-inert or even bioactive materials can also be processed by injection molding. New biodegradable composite materials have a great perspective for future implants. Currently biodegradable composites are under development to design materials with a well defined degradation rate. First results have shown the potential that the injection molding process is a very well suited shaping process for these materials.
- AM technologies are becoming more and more important to produced customized medical components or small series components. New freedom in product design also creates opportunities to integrate more functionality into components. The most important production processes for CoCr alloys and Ti alloys are SLM and EBM. For both processes the qualification of new powders is a further step towards the broader acceptance of AM technologies as advanced manufacturing processes in more and more medical applications.
Conclusion
Fig. 4.SEM documentation of the grain refinement of stainless steel a) without addition of nanopowder and b) after using 33 % nanopowder, sintered at 1200°C.
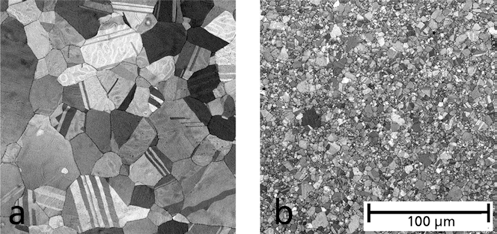

Fig. 7.AM study for material combinations of dense and porous areas of a component.: (a) dense shell, porous core, (b) dense core, porous shell.


Table 1.Comparison of Grade 5 standard and properties of MIM Ti-6Al-4V
Table 2.Comparison of properties of Ti-6Al-V processed by SLM and by MIM
| Ti-6Al-4V | SLM | MIM |
|---|---|---|
|
|
||
| Oxygen content [%] | ≤ 0.15 | < 0.25 |
| Carbon content [%] | - | < 0.07 |
| Nitrogen content [%] | ≤ 0.07 | < 0.05 |
| Yield Strength [N/mm2] | 1000 | 825 |
| Tensile strength [N/mm2] | 1100 | 940 |
| Elongation [%] | 8 | 9 |
- 1. P Imgrund, F Petzoldt and V Friederici, Proc Euro PM. (2008) 2 305.
- 2. T Ebel, PIM Int. (2008) 2 21.
- 3. P Imgrund, S Hein, V Friederici and N Salk, PIM Int. (2010) 4 49.
- 4. V Friederici, A Bruinink, P Imgrund and S Seefried, Proc. PM World Congress. (2010) 5 785.
- 5. D Auzène, C Mallejac, C Demangel, F Lebel, J Duval, P Vigneron and J Puippe, Proc. Euro PM. (2011) 2 441.
- 6. I Tsyganov, M Matiz and E Wieser, Surf. Coat. Tech. (2005) 200 1041.Article
- 7. A Bruinink, J Kaiser and D Meyer, Adv. Eng. Mater. (2005) 7 411.Article
- 8. F Petzoldt, C Aumund-Kopp and I Uckelmann, Proc. Hagener Symposium. (2008) 24 181.
- 9. C Aumund-Kopp, To be published in Proc. of Fraunhofer DDMC. (2014.
- 10. E Marin, S Fusi, M Pressacco, L Paussa and L Fedrizzi, J. Mech. Behav. Biomed. Mater. (2010) 3 373.Article
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Enhancing corrosion resistance of Ti-based amorphous alloy powders via misch metal addition
Yeon Joo Lee, Hyokyung Sung, Jae Bok Seol, Kisub Cho, Hwi Jun Kim, Hyunjoo Choi
Powder Metallurgy.2025; 68(3): 230. CrossRef - Spontaneous Formation of Titanium Nitride on the Surface of a Ti Rod Induced by Electro-Discharge-Heat-Treatment in an N2 Atmosphere
W.H. Lee, Y.H. Yoon, Y.H. Kim, Y.K. Lee, J.Y. Kim, S.Y. Chang
Archives of Metallurgy and Materials.2017; 62(2): 1281. CrossRef
Advanced PM Processes for Medical Technologies


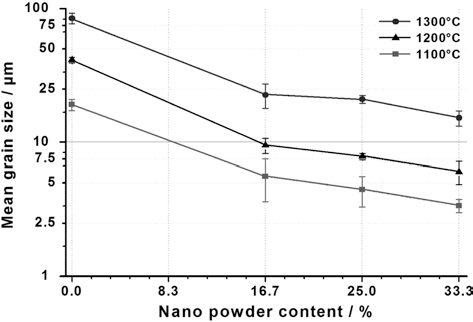
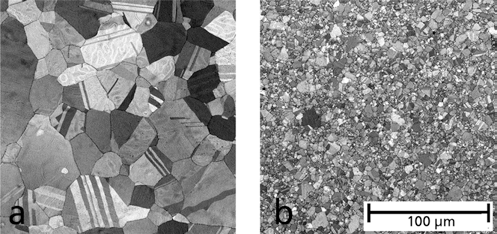
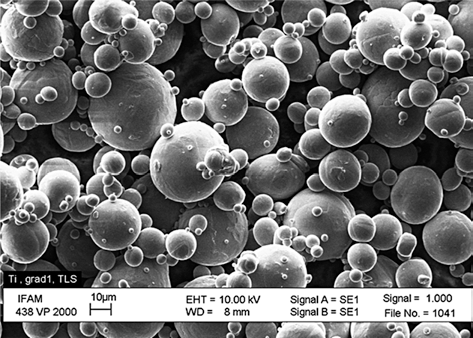
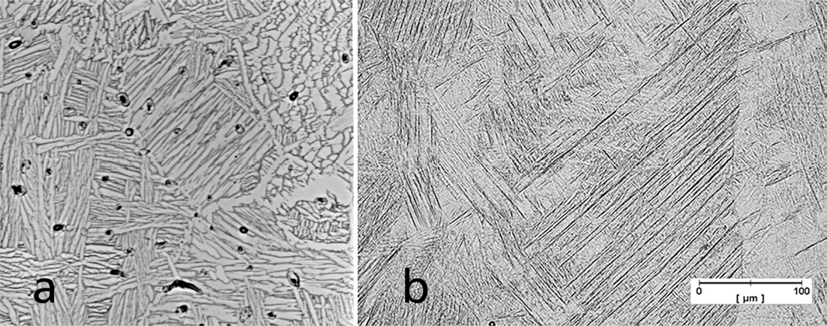
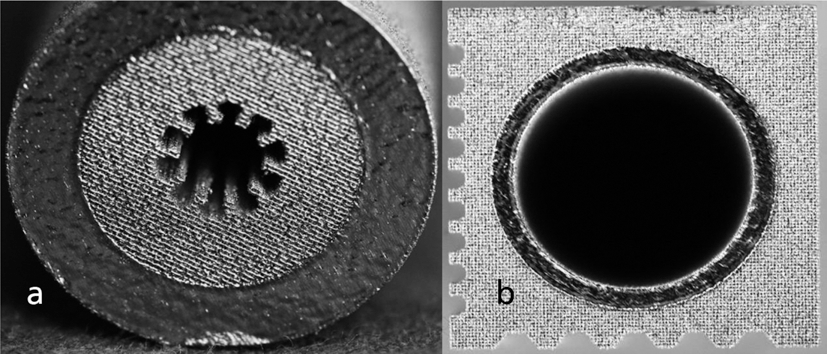
Fig. 1.
Evaluation of density and microstructure of different Ti powders and binders.
Fig. 2.
Grain size depending on nanopowder content at different sintering temperatures.
Fig. 3.
Grain size depending on nanopowder content at different sintering temperatures.
Fig. 4.
SEM documentation of the grain refinement of stainless steel a) without addition of nanopowder and b) after using 33 % nanopowder, sintered at 1200°C.
Fig. 5.
SEM picture of gas atomized titanium powder.
Fig. 6.
Comparison of the microstructure of (a) injection molded and (b) laser melted Ti-6Al-4V.
Fig. 7.
AM study for material combinations of dense and porous areas of a component.: (a) dense shell, porous core, (b) dense core, porous shell.
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
Fig. 7.
Advanced PM Processes for Medical Technologies
| Ti-6Al-4V | Grade 5 | MIM |
|---|---|---|
|
|
||
| Oxygen content [%] | ≤ 0.2 | < 0.25 |
| Carbon content [%] | ≤ 0.1 | < 0.07 |
| Nitrogen content [%] | ≤ 0.05 | < 0.05 |
| Yield Strength [N/mm2] | 830-924 | 825 |
| Tensile strength [N/mm2] | 900-993 | 940 |
| Elongation [%] | 14 | 9 |
| Ti-6Al-4V | SLM | MIM |
|---|---|---|
|
|
||
| Oxygen content [%] | ≤ 0.15 | < 0.25 |
| Carbon content [%] | - | < 0.07 |
| Nitrogen content [%] | ≤ 0.07 | < 0.05 |
| Yield Strength [N/mm2] | 1000 | 825 |
| Tensile strength [N/mm2] | 1100 | 940 |
| Elongation [%] | 8 | 9 |
Table 1.
Comparison of Grade 5 standard and properties of MIM Ti-6Al-4V
Table 2.
Comparison of properties of Ti-6Al-V processed by SLM and by MIM
Table 1.
Table 2.
TOP
 KPMI
KPMI

 Cite this Article
Cite this Article







