Articles
- Page Path
- HOME > J Korean Powder Metall Inst > Volume 21(4); 2014 > Article
-
ARTICLE
- Production of Porous Metallic Glass Granule by Optimizing Chemical Processing
- Song-Yi Kim1, Bo-Kyung Guem1, Min-Ha Lee1, Taek-Soo Kim1, Jurgen Eckert2, Bum-Sung Kim1,*
-
Journal of Korean Powder Metallurgy Institute 2014;21(4):251-255.
DOI: https://doi.org/10.4150/KPMI.2014.21.4.251
Published online: July 31, 2014
- * Corresponding author: Bum-Sung Kim, TEL: +82-32-850-0286, FAX: +82-32-850-0304, E-mail: bskim15@kitech.re.kr
• Received: July 15, 2014 • Revised: August 12, 2014 • Accepted: August 19, 2014
© Korean Powder Metallurgy Institute
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,064 Views
- 2 Download
- 1 Crossref
Abstract
- In this study, we optimized dissolution the dissolution conditions of porous amorphous powder to have high specific surface area. Porous metallic glass(MG) granules were fabricated by selective phase dissolution, in which brass is removed from a composite powder consisting of MG and 40 vol.% brass. Dissolution was achieved through various concentrations of H2SO4 and HNO3, with HNO3 proving to have the faster reaction kinetics. Porous powders were analyzed by differential scanning calorimetry to observe crystallization behavior. The Microstructure of milled powder and dissolved powder was analyzed by scanning electron microscope. To check for residual in the dissolved powder after dissolution, energy dispersive X-ray spectroscory and elemental mapping was conducted. It was confirmed that the MG/brass composite powder dissolved in 10% HNO3 produced a porous MG granule with a relatively high specific surface area of 19.60 m2/g. This proved to be the optimum dissolution condition in which both a porous internal granule structure and amorphous phase were maintained. Consequently, porous MG granules were effectively fabricated and applications of such structures can be expanded.
- Porous materials have been utilized in appliances and electronic equipment wherein fluid permeability and large reaction area are required. Porous metals can also be applied in a variety of fields such as filters, catalyst supports, and electrode materials, provided they have sufficient mechanical properties and chemical stability [1-3]. To realize these unique porous metal structures, it is important to select both a mechano-chemically stable material system and a reliable manufacturing process. Porous metallic glasses (MG) with a high surface area are a novel material for such purposes, and owing to their unique porous granule structure, combined with desirable strengths at a relatively low density, they have been investigated for applications such as energy absorbers and fuel cell membranes [4-9]. Several methods for the fabrication of porous MG structures have previously been reported, with Wada et al. [10,11] producing a Pd-based MG foam by water-quenching a melt mixed with solid NaCl salt, and by vitrifying a melt under pressurized hydrogen. Brothers and Dunand [12,13] also reported on the fabrication of a Zr-based bulk MG foam. Of particular importance to porous MG is maintaining the amorphous phase and porous internal granule structure, as final applications are reliant on a high specific surface area. Although the results of these studies are quite promising, they have not met industrial demands owing to the complexity of the process and difficulty of mass production.
- In this study, we are the first to report on the optimization of chemical processing by selective phase dissolution of porous MG from powdered granules of Ni59Zr20Ti16Si2Sn3. Selective dissolution is a process wherein one base-metallic phase is selectively removed by means of an acid from base-metal and noble-MG composite granules. It is therefore important to understand dissolution behaviors in a powder state, as MG can be produced from both powders and sintered bodies. This composition of Ni59Zr20Ti16Si2Sn3 exhibits beneficial properties such as a relatively high glass forming ability, chemical stability, and a fracture strength of 2700 MPa [14].
Introduction
- MG(Ni59Zr20Ti16Sn3Si2) was produced as a fully amorphous spherical powder by high-pressure Ar gas atomization, using a previously melted master alloy [16]. A composite powder containing 60 vol.% MG and 40 vol.% powdered brass (Cu65Zn35) was made by conventional ball milling using Retsch PM400 planetary equipped with hardened steel balls and vials followed by cryogenic ball milling to refine the layer spacing under N2 atmosphere [17]. Vial charging was performed under purified argon atmosphere (less than 1 ppm O2 and H2O). The size of the MG powder used for this study was less than 90 μm, with the brass powder used as secondary phase similarly sized at less than 90 μm. Chemical dissolution was achieved by two acid solutions (H2SO4, HNO3) to selectively remove the secondary phase from MG composites. A α-brassis selected as the base metal owing to the relative ease with which it forms composite granules with MG during milling. Details of the composite powder synthesis are described elsewhere [15]. In a randomly selected 4 g sample of ball-milled composite powder, acid-soluble brass phases were removed by the selective dissolution process. Two types of acids H2SO4 and HNO3 were chosen for this purpose, with concentrations of 10%, 20%, and 30% and a dissolution time of 24 h at room temperature. The resulting porous MG granule was extracted by filtration and rinsed ten times to remove any remaining H2SO4 or HNO3 solution, and the filtered powder was then dried in 100°C for 24 h. Weight loss for the dried porous powder was determined to investigate the effects of the different acid concentrations. The porous MG granule was characterized using X-ray diffraction (XRD; Philips APD 3520) with monochromatic Cu-Kα radiation. The microstructure of the porous MG granule was characterized by scanning electron microscopy (SEM; JEOL 5910LV). The thermal stability of the porous MG granule was determined by differential scanning calorimetry (DSC; Perkin-Elmer Pyris-7) at 40°C/min heating rate under a continuous flow of purified argon. The specific surface area of the porous MG granule was determined using the Brunauer-Emmett-Teller method (BET; Adsortrac DN-04).
Experimental
- Fig. 1 shows the weight loss for the porous MG granules with increasing concentrations of H2SO4 and HNO3. The reaction between H2SO4 and brass is well known, and results in the formation of Cu2SO4 (cupric sulfate) and ZnSO4 (zinc sulfate). However, when brass is reacted with HNO3, the end products are Cu(NO3)2 (cupric nitrate) and Zn(NO3)2 (zinc nitrate). Therefore, if the brass phase is completely removed by selective dissolution, the resultant granule should contain 60% MG by volume for a total weight of 2.1 g. In the case of in H2SO4, the weight loss increased from 5% to 8%, and then to 8.1%, with increasing acid concentrations. These results suggest that over 90% of the original brass phase remained in the composite granules. By increasing the HNO3 concentration to 20% and 30%, the weight losses were determined to be 58% and 60%, respectively. In contrast, the weight loss for the porous MG granule dissolved in 10% HNO3 solution corresponded to 15%, representing a significant reduction in the amount of brass remaining. This demonstrates that the selective dissolution behavior is strongly dependent on the acid conditions, with HNO3 superior to H2SO4 for the dissolution of brass. HNO3 may be suitable for use in the selective dissolution process.
- Weight loss rate after 24 h for porous MG granules dissolved in H2SO4 and HNO3 as a function of concentration.
- The microstructures of the porous MG granules were observed using SEM (back-scattered electrons), as shown in Fig. 2, to identify reaction conditions that maintained the granular structure. Light-gray regions indicate an MG phase, with brass phases represented by dark gray regions, as can be seen in Figs. 2(a)-(c). In the case of H2SO4, a residual brass phase is present, with the amount of this phase decreasing with increasing acid concentrations. Pores with small volumes are found in those areas where brass phases have been removed, with the pore volume increasing slightly with increasing acid concentration.
- Scanning electron microscopy (back-scattered electron) images of porous powder dissolved in (a) 10%, (b) 20%, and (c) 30% H2SO4; and dissolved in (d) 10%, (e) 20%, and (f) 30% HNO3.
- In the case of HNO3 (Figs. 2(d)-(f)), however, the microstructures of the porous MG granules are found to have a more many pores than with H2SO4 at low concentration. A porous MG granule dissolved in 10% HNO3 solution, as shown Fig. 2(d), maintains a porous internal structure with a fine layer spacing, and would be expected to have a high specific surface area. In the case of 20% HNO3 (Fig. 2e), layer spacing widens and pores expand. Finally, with 30% HNO3, the interface of MG becomes flat owing to MG dissolution. Consequently, although some residual brass still remains, 10% HNO3 is considered to produce the most ideal microstructure.
- Fig. 3 shows XRD results obtained from the initial composite granule and granules treated with H2SO4 (Fig. 3a) and HNO3 (Fig. 3b) to confirm the presence of residual brass and reaction products. The initial composite powder has peaks at 43°, 50°, and 73°, indicating the presence of the α-brass phase. After dissolution in H2SO4 (Fig. 3a), the granules exhibit brass peaks that were expected based on the weight loss rate results (Fig. 1) and morphology studies (Fig. 2). For the porous granule dissolved in 10% HNO3 (Fig. 3b), peaks for both brass and an unknown phase were detected, with this unknown phase possibly being reaction products of solids and HNO3. It is also interesting to note that porous MG in 10% HNO3 has a narrow pore structure, which cannot easily remove reaction products with the rinsing procedures that were used. Porous granules dissolved in 20% and 30% HNO3 show the typical halo peak of MG, without any evidence for the presence of unreacted brass phases.
- X-ray diffraction patterns for porous MG granules dissolved in different concentrations of (a) H2SO4 and (b) HNO3.
- The amorphous powder shows a crystallization temperature (Tx) followed by a single exothermic event. The ΔTx defined as ΔTx = Tx-Tg is supercooled liquid region of amorphous powder. Thermal analysis results (Figs. 4(a), (b)), were obtained from porous granules dissolved in H2SO4 and HNO3 during continuous heating at a rate of 40 °C/min. The enthalpy for the initial MG powder is -57.8 J/g[15], with the exothermic peak increasing this to -18.1, -19.8, and -19.9 J/g with increasing H2SO4 concentration, owing to residual α-brass. As the weight of this residual brass decreases slightly (Fig. 1), the enthalpy of the porous MG granule increases. Similarly, the exothermic peak increases to -26.5, -42.8, and -49.9 J/g with an increasing HNO3 concentration. The enthalpy of the porous granules should be equal to that of initial MG powder in the case of 20% and 30% HNO3, as brass was found to be fully removed in both instances. However, this was not the case, as partial composition of MG is altered by reaction with the relatively high concentration acids. As the results suggest in Fig. 1, 121% and 126% of the brass is dissolved in HNO3, and so it is evident that 21% and 26% of MG must be dissolved. Although some brass remains, the MG granule dissolved in 10% HNO3 maintained the original porous structure and the basic characteristics of MG. Though a reaction to acid solutions occurs simultaneously with both MG and brass phases, the rate of dissolution is observed to be much slower with MG phases.
- Differential scanning calorimetry traces (40°C/min) for porous powder dissolved in (a) H2SO4 and (b) HNO3 aqueous solutions of various concentrations.
- Fig. 5 shows the specific surface area of porous granules as determined by the BET method. The porous granules dissolved in 10%, 20%, and 30% H2SO4 have relatively low specific surface areas of 0.04, 0.06, and 0.09 m2/g, respectively. These results correspond with those of the weight loss rate (Fig. 1), SEM (Fig. 2), XRD, and DSC (Fig. 3). With the porous granule dissolved in 10% HNO3, however, the maximum specific surface area of 19.60 m2/g is a relatively high value obtained from a powder state for the first time. These results are near in value to those obtained in a study by Lee [2], wherein a porous MG foam was made by selective dissolution of an extruded bulk comprising composite powder (Cu-based MG/40 vol% Cu) to give a specific surface area of 23.5 m2/g. For those powders treated with 20% and 30% HNO3, the specific surface area decreased from 7.5 to 2.3 m2/g with increasing concentration, as the MG surface becomes flat and broken owing to over-etching.
Results and Discussion
Fig. 1.
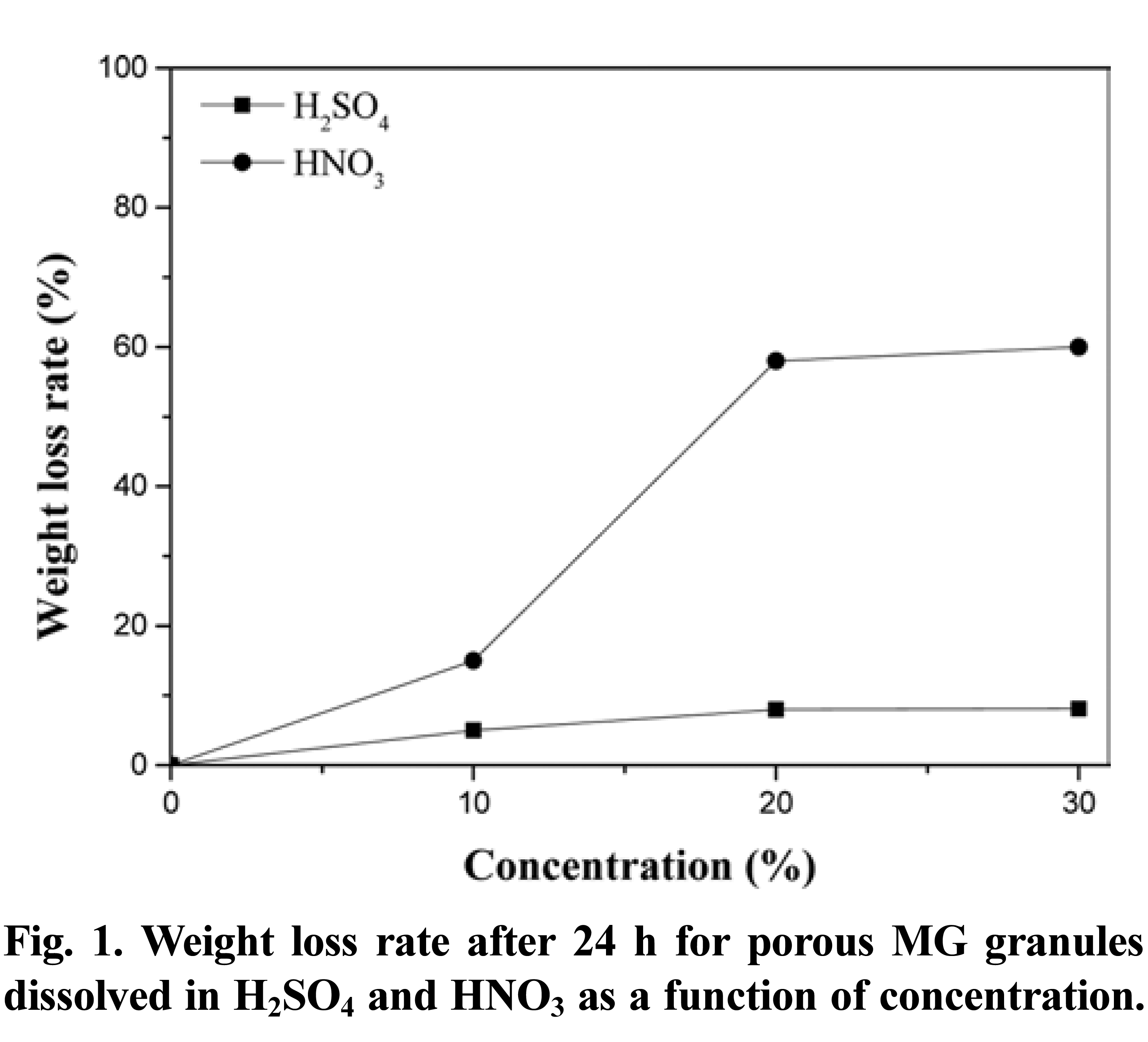

Fig. 2.


Fig. 3.
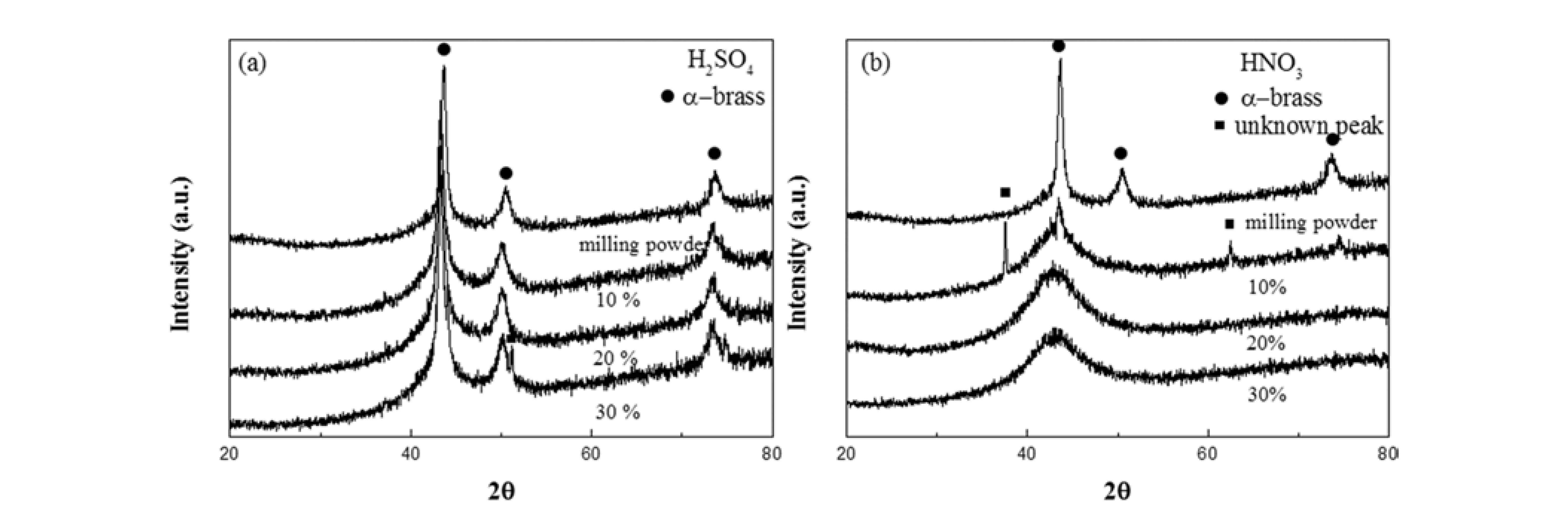

Fig. 4.


- To produce a porous granule, a composite powder (MG/40 vol.% brass) was prepared by conventional ball milling for 20 h followed by 1 h of cryogenic ball milling to refine the layer space. An á-brass phase was dissolved by selective phase dissolution in an acid solution (H2SO4, HNO3) with marginal dissolution of the MG phase. The reaction between HNO3 and brass was found to be superior to that between H2SO4 and brass under all experimental conditions, insofar as the rate of reaction is concerned. As shown by the SEM images, granules dissolved in H2SO4 and 10% HNO3 have morphology characteristics suitable to the formation of porous granule structures. The granule dissolved in 10% HNO3 also had a relatively high specific surface area (19.6 m2/g); this is the first time such a high value has been obtained from a powder state. To summarize the microstructural results, a granule dissolved in 10% HNO3 is chemically stable and maintains a porous internal granule structure, although very little brass remains. The layer space of a granule dissolved in 10% HNO3 was also constant, and for these reasons, is ideal for ensuring optimal porosity.
Conclusions
- 1. A. H Brothers, R Scheunemann, J. D DeFouw and D. C Dunand, Scripta Mater. (2005) 52 335.Article
- 2. M. H Lee and D. J Sordelet, Scripta Mater. (2006) 55 947.Article
- 3. J Erlebacher and K Sieradzki, Scripta Mater. (2003) 49 991.Article
- 4. H. A Bruck, T Christman, A. J Rosakis and W. L Johnson, Scr Metall Mater. (1994) 30 429.Article
- 5. J. S Kim, M. H Lee, D. H Kim, U Kühn and J Eckert, J. Rev Metal. (2012) 109 11.Article
- 6. C. J Gilbert, R. O Ritchie and W. L Johnson, Appl Phys Lett. (1997) 71 476.ArticlePDF
- 7. A Inoue, Acta Mater. (2000) 48 279.Article
- 8. P. S Liu and K. M Liang, J. Mater Sci. (2001) 36 5059.Article
- 9. S. Y Yang, I. R Hwang, Y Kim, J. K Kim, K. S. S. K Jang and T. P Russell, Adv Mater. (2006) 18 709.Article
- 10. T Wade and A Inoue, Mater Trans. (2003) 44 2228.Article
- 11. T Wade, A Inoue and A. L Greer, Appl Phys Lett. (2005) 86 251.
- 12. A. H Brothers and D. C Dunand, Appl Phys Lett. (2004) 84 1108.ArticlePDF
- 13. A. H Brothers and D. C Dunand, Adv Mater. (2005) 17 484.Article
- 14. J. K Lee, D. H Bae, S Yi, W. T Kim and D. H Kim, J Non-Cryst Solids. (2004) 333 212.Article
- 15. S. Y Kim, B. K Guem, M. H Lee and B. S Kim, J Kor Powd Met Inst. (2013) 20 36.
- 16. D. H Bae, M. H Lee, D. H Kim and D. J Sordelet, Appl Phys Lett. (2003) 83 2312.ArticlePDF
- 17. J. Y Kim, S Scudino, U Kühn, B. S Kim and M. H Lee, J. Eckert: Metals. (2012) 2 79.Article
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Enhanced wear resistivity of a Zr-based bulk metallic glass processed by high-pressure torsion under reciprocating dry conditions
Soo-Hyun Joo, Dong-Hai Pi, Jing Guo, Hidemi Kato, Sunghak Lee, Hyoung Seop Kim
Metals and Materials International.2016; 22(3): 383. CrossRef
Production of Porous Metallic Glass Granule by Optimizing Chemical Processing
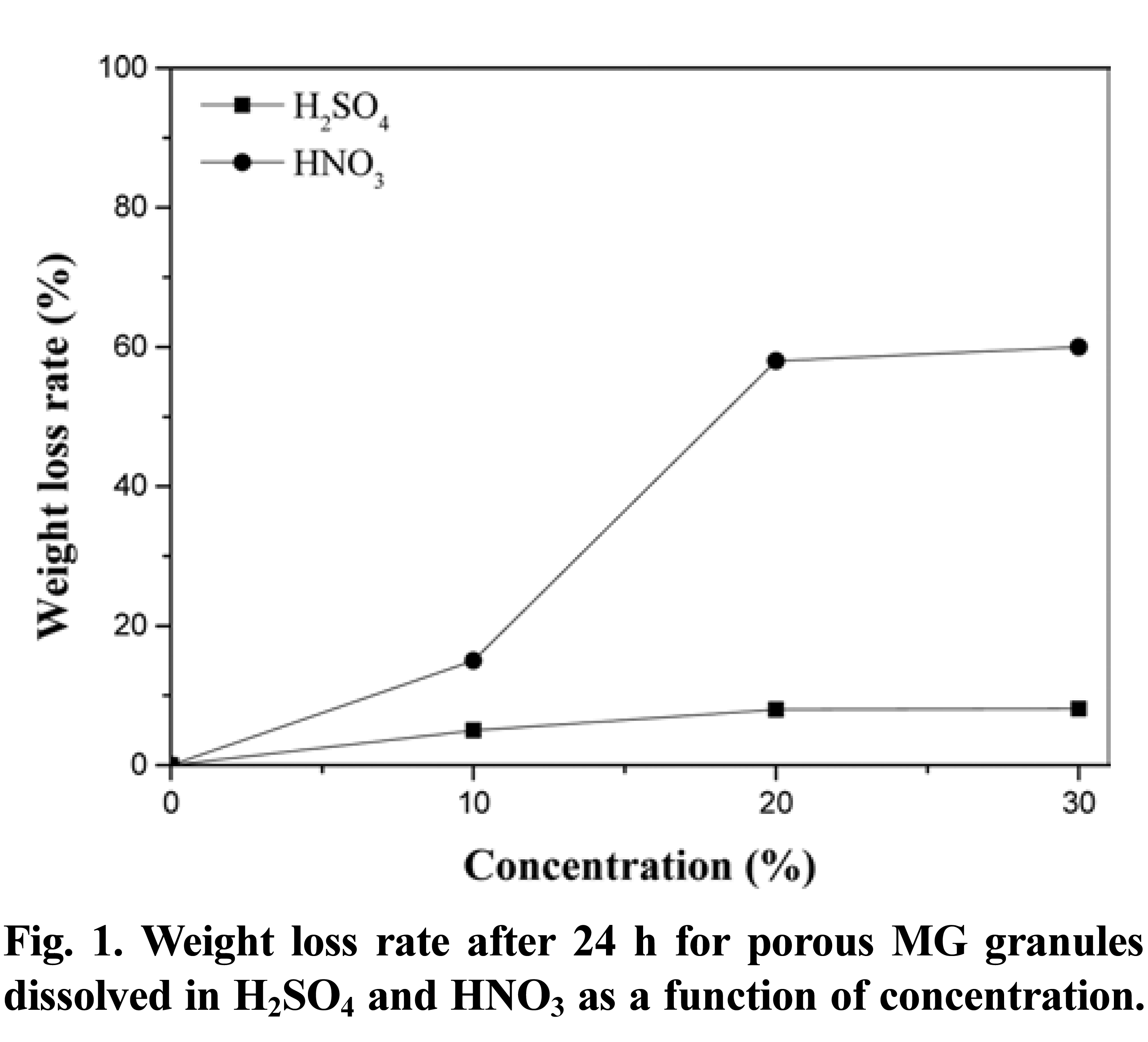
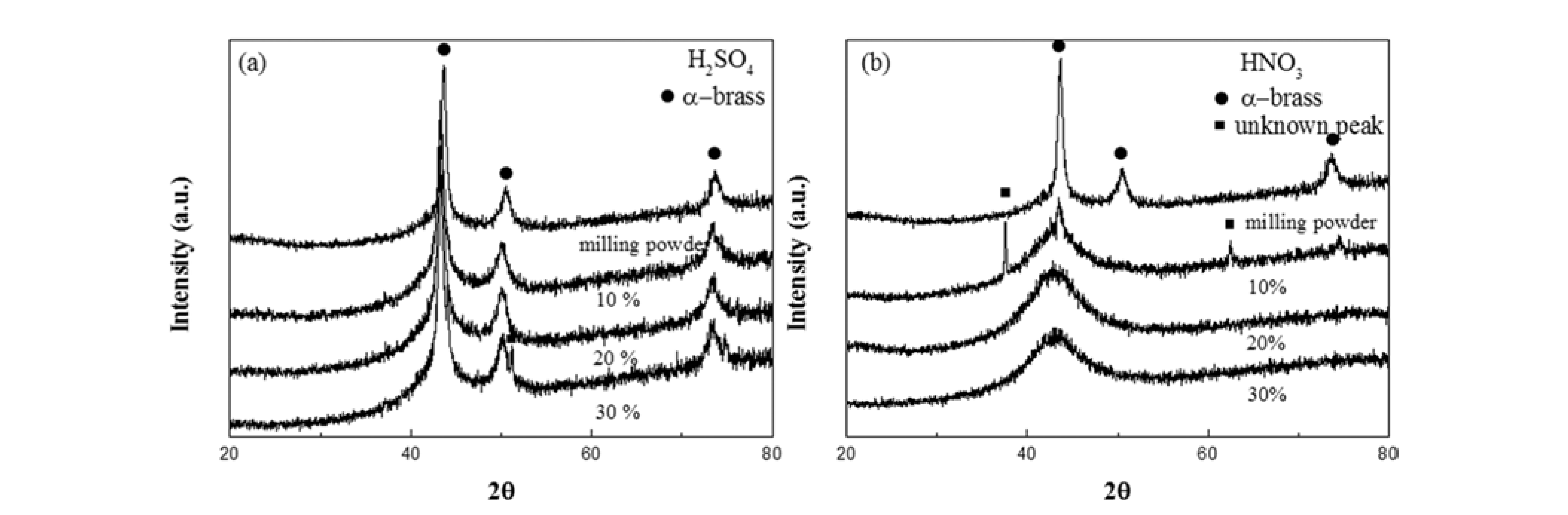
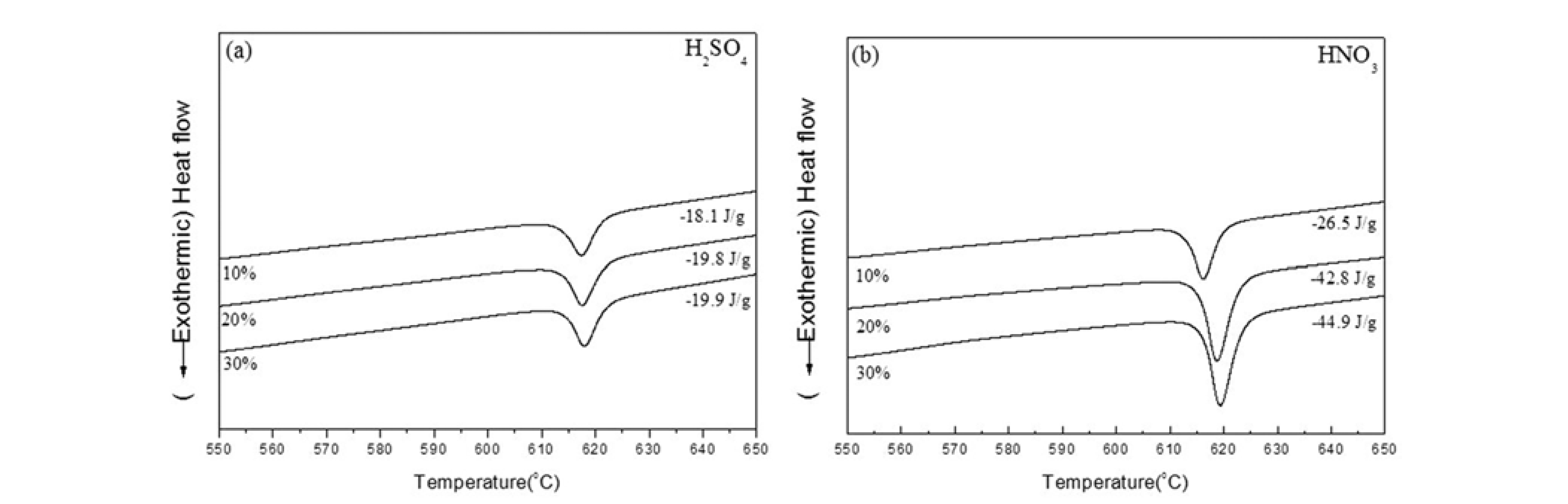
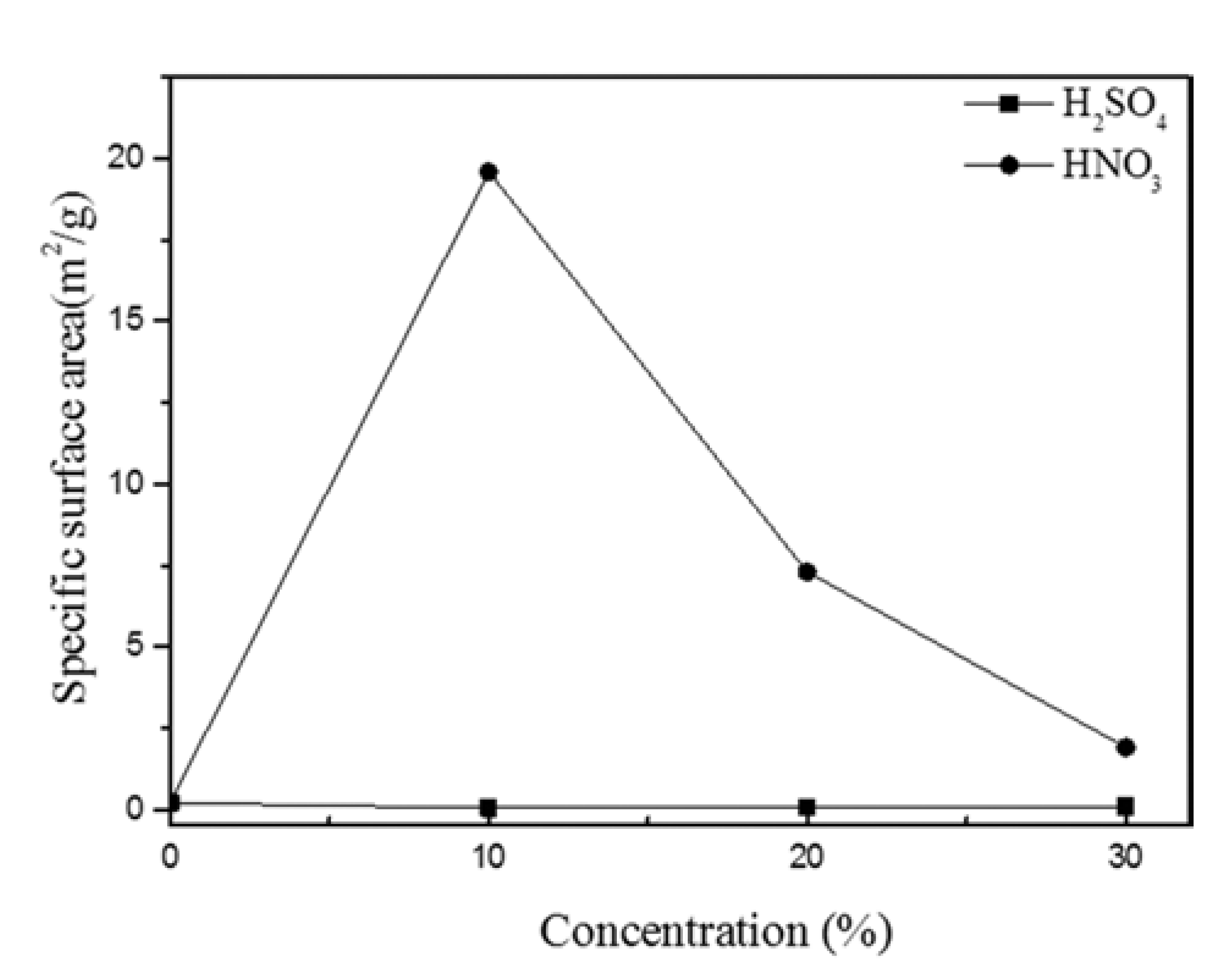
Fig. 1.
Weight loss rate after 24 h for porous MG granules dissolved in H2SO4 and HNO3 as a function of concentration.
Fig. 2.
Scanning electron microscopy (back-scattered electron) images of porous powder dissolved in (a) 10%, (b) 20%, and (c) 30% H2SO4; and dissolved in (d) 10%, (e) 20%, and (f) 30% HNO3.
Fig. 3.
X-ray diffraction patterns for porous MG granules dissolved in different concentrations of (a) H2SO4 and (b) HNO3.
Fig. 4.
Differential scanning calorimetry traces (40°C/min) for porous powder dissolved in (a) H2SO4 and (b) HNO3 aqueous solutions of various concentrations.
Fig. 5.
Specific surface area of powder dissolved in various concentrations of H2SO4 and HNO3 aqueous solutions.
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Production of Porous Metallic Glass Granule by Optimizing Chemical Processing
TOP
 KPMI
KPMI

 Cite this Article
Cite this Article





