Articles
- Page Path
- HOME > J Korean Powder Metall Inst > Volume 21(2); 2014 > Article
-
ARTICLE
- Coating of Cobalt Over Tungsten Carbide Powder by Wet Chemical Reduction Method
- Hyun-Seon Hong, Jin-Ho Yoon*
-
Journal of Korean Powder Metallurgy Institute 2014;21(2):93-96.
DOI: https://doi.org/10.4150/KPMI.2014.21.2.93
Published online: March 31, 2014
- *Corresponding Author : Jin-Ho Yoon, TEL: +82-31-330-7481, FAX: +82-31-330-7113, E-mail: yjh6373@iae.re.kr
• Received: February 25, 2014 • Revised: April 21, 2014 • Accepted: April 25, 2014
© Korean Powder Metallurgy Institute
- 1,533 Views
- 8 Download
- 3 Crossref
Abstract
- Cobalt coated tungsten carbide-cobalt composite powder has been prepared through wet chemical reduction method. The cobalt sulfate solution was converted to the cobalt chloride then the cobalt hydroxide. The tungsten carbide powders were added in to the cobalt hydroxide, the cobalt hydroxide was reduced and coated over tungsten carbide powder using hypo-phosphorous acid. Both the cobalt and the tungsten carbide phase peaks were evident in the tungsten carbide-cobalt composite powder by X-ray diffraction. The average particle size measured via scanning electron microscope, particle size analysis was around 380 nm and the thickness of coated cobalt was determined to be 30~40 nm by transmission electron microscopy.
- Development of various tough materials has increased need for the material machining technique, accordingly, demands for cutting tools such as cemented carbide tools are also rapidly increasing. A cemented carbide tool has excellent hardness and deflective strength at high temperature. Thus, the cemented carbide tools (like tungsten carbide- cobalt (WC-Co) composite) are widely used in the industries for precise machining due to its superior wear resistance, and machinability.
- The mechanical characteristics of the cemented carbide material mostly depend on the process like sintering and characteristics of raw materials like shape, size, distribution and thickness of cobalt (Co) coating of tungsten carbide- cobalt (WC-Co) composite. Though the tungsten carbide (WC) raw material is being used in industry, the WC-Co composite has significantly better mechanical properties over WC. In the WC-Co composite size and thickness of the Co coating plays a vital role. Therefore, the technique, which produces nano-sized Co, plays an essential role in improving the properties of the cemented carbide tools. Also, the Co can be used in various forms in the manufacturing process of the WC tools due to its superior moldability, particle size, shape, and chemical purity. The Co has been the most commonly used material because it can be effectively compacted up to a theoretical density by hot pressing at relatively low temperature and pressure conditions.
- To improve the properties of the WC tools, synthesis of WC-Co composite by various route such as hydrothermal, electro-thermal explosion directional spraying, spark plasma sintering, liquid phase sintering, hot press sintering has been reported [1-6]. In our process very simple and easier process been developed for coating of 30-40 nm Co, which further can be scaled up for commercial production.
Introduction
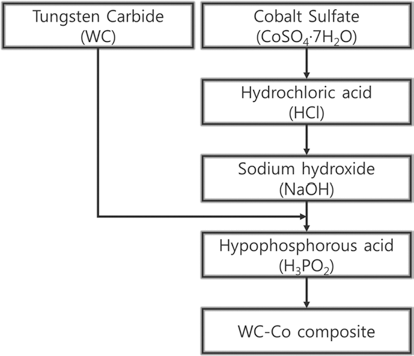
- The experiment was performed to the following process to the synthesis of WC-Co submicron composite similar to our process report elsewhere [7]. Fig. 1 depicts the process flow sheet for synthesis of WC-Co composite. A cobalt sulfate (CoSO4∙7H2O) solution was dissolved in deionized (DI) water in a reactor, and the cobalt sulfate solution was substituted with cobalt chloride (CoCl2) adding 35% hydrochloric acid (HCl) under agitation for 10 min. The CoCl2 was again substituted as cobalt hydroxide (Co(OH)2) by sodium hydroxide (NaOH). The WC powder was added and allowed to diffuse so that the Co(OH)2 can evenly adhere to the surface of WC. Finally the reductant hypophosphoric acid (H3PO2) was added to reduce the Co(OH)2 to the Co over the WC surface. The synthesized WC-Co composite powder was washed several times using DI water and ethanol, and was finally dried using a vacuum desiccator. The synthesized WCCo composite powder was characterized by X-ray diffraction (XRD, ATX-G, Rigaku), scanning electron microscope (SEM, Nova Nano 200, FEI) and by transmission electron microscopy (TEM, JEM 4000FX, JEOL). The particle size and zeta potential of the WC-Co were analyzed through zeta potential and particle size analyzer (ELSZ-2, Photal).
Experiment
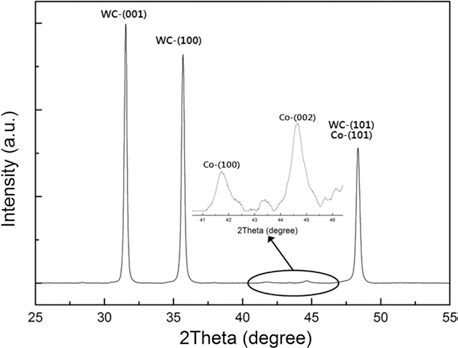
- The crystal structure of the WC-Co composite powder was analyzed using XRD, and Fig. 2 shows the result. As shown in XRD, the primary (main) peaks for the WC-Co composite powder, i.e. (001), (100), and (101), (JDPDS 51-0939), and peaks (100), (101) and (002) for Co (JCPDS 05-0727) were identified, when the scanned angle 2θs were in between 40-48° radian. The peaks (101) for WC and Co were superimposed, hardly can be observed directly.
- Fig. 3(a) and (b), the SEM show the surfaces of WC and the WC-Co composite powder observed. The observed particle size was about 100-700 nm for WC and the WCCo composite powder, respectively. The distribution of each element was examined via energy-dispersive spectrometry (EDS) to identify the elements of the WC-Co composite powder. Fig. 3(c) and (d) show the EDS mapping of the WC-Co composite powder. Both tungsten (W), the element of WC, and Co are evenly distributed, but distribution for W is more intense than Co. Fig. 3(e) shows the element distribution of the WC-Co composite powder observed via line scanning. The W was found to be distributed relatively near the central region, and the Co was found to be distributed relatively near the edge region, reasonably concluded that that the surface of WC is coated with Co.
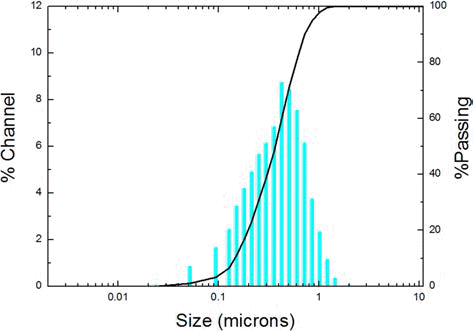
- Fig. 4 shows the particle distribution of the WC-Co composite powder, was examined by PSA. The average particle size was 381 nm, and D10 and D90 were 149 and 720 nm, respectively. Thus, the dispersibility of WC-Co is 4.8. The particle distribution of the composite powder is consistent as observed in SEM.
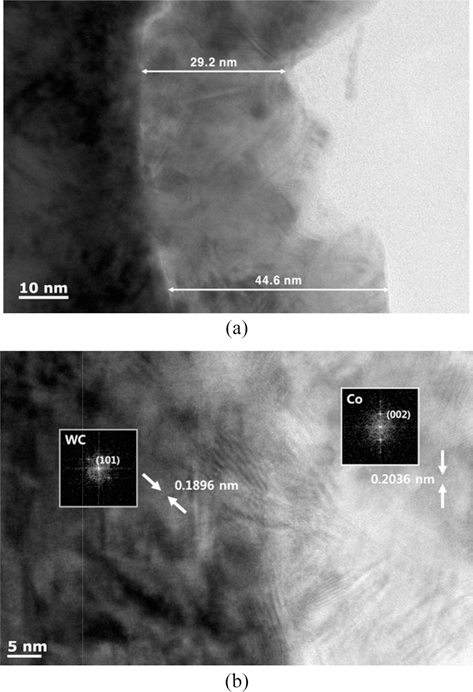
- To figure out the coating condition of the WC-Co composite powder, the cross-section of the WC-Co composite powder was cut using FIB, and the distribution of Co was examined via TEM. As shown in Fig. 5(a), the surface of WC is uniformly coated with the Co, and the thickness of the Co coating is 30-40 nm. The inter-planar spacing of each elements of WC-Co was calculated via FFT, and Fig. 5(c) shows the result. The center and edge regions of the WC-Co composite powder were arbitrarily established, and the SEAD pattern was presented. Each interplanar spacing was measured using the calculated result. In the WC area, which is the central region, a 0.1896 nm inter-planar spacing was calculated, which is consistent with (101) superimposed peak of the XRD analysis presented Fig. 2. In the Co area, which is the edge region, a 0.2036 nm inter-planar spacing was calculated, which is also consistent for the (002) peak of Co. In the WC-Co composite powder, the boundary of WC (center region) is uniformly coated with Co, with a thickness of 30-40 nm.
- In general, the redox reaction of metal spontaneously feasible when the total electrochemical potential (ΔE0) has a positive value, and the reduction-oxidation half shell reactions for Co(OH)2 and H3PO3, respectively in basic solution can be expressed as follow:
- where E0 is the standard electrode potential. If the E0 of the reduction reaction is lower than the E0 of oxidation reaction, the ΔE of oxidation-reduction reaction has a positive value, and a spontaneous reaction occurs. Therefore, Co(OH)2 is spontaneously reduced to nano cobalt powder by reducing the agent, H3PO3, at room temperature while the reducing agent, H3PO3 experiences the oxidation reaction. The reaction of Co(OH)2 and H3PO3 can be expressed as the following reaction formula:
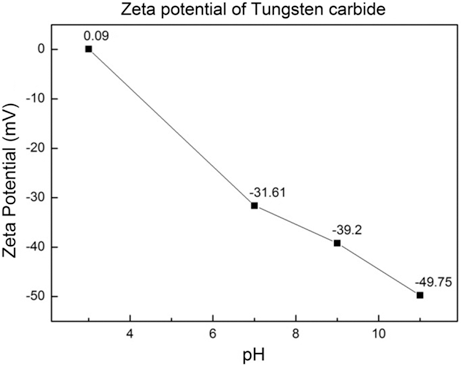
- To figure out whether the reduction reaction of Co is feasible at the surface of WC, the zeta potential of WC was measured depending on the pH, and Fig. 6 shows the result. At pH 3, which is an acidic condition, the zeta potential was 0.09 mV, but as the pH was increased to 7, 9, and 11, the zeta potential was decreased to -31.61, -39.2, and -49.75 mV. It is enough energy for Co2+, which is the cation of Co(OH)2, to adhere to the surface of WC, and through this result, Co was found to be uniformly formed on the surface of WC. This is consistent with the fact that it is not that Co exists as powder by independent reduction.
Results and Discussion
- The WC-Co composite powder was successfully synthesized at room temperature by wet chemical reduction method. The cobalt sulfate solution was converted to the cobalt chloride then the cobalt hydroxide. The tungsten carbide powders were added in to the cobalt hydroxide, the cobalt hydroxide was reduced and coated over tungsten carbide powder using hypophosphoric acid. The WC-Co composite powder was nanocrystalline materials and the average particle size was around 380 nm. The surface of WC uniformly coated with the Co, and the thickness was 30~40 nm. The zeta potential showed -49.75 mV, when the pH is 11. It is enough energy for Co2+ to adhere to the surface of WC, Co was found to be uniformly formed on the surface of WC. The synthesized WC-Co composite powder is expected to improve the characteristic of the cemented-carbide tool.
Conclusions
-
Acknowledgements
- This work was supported by a grant from the Fundamental R&D Program for Core Technology of Materials (10037201) funded by the Ministry of Trade, Industry and Energy, Republic of Korea.
Acknowledgements
Fig. 3.The SEM of the WC-Co composite powder. (a) the pure WC, (b) WC-Co, EDS analysis of (c) W, EDS analysis of (d) Co, and (e) Line scanning analysis.
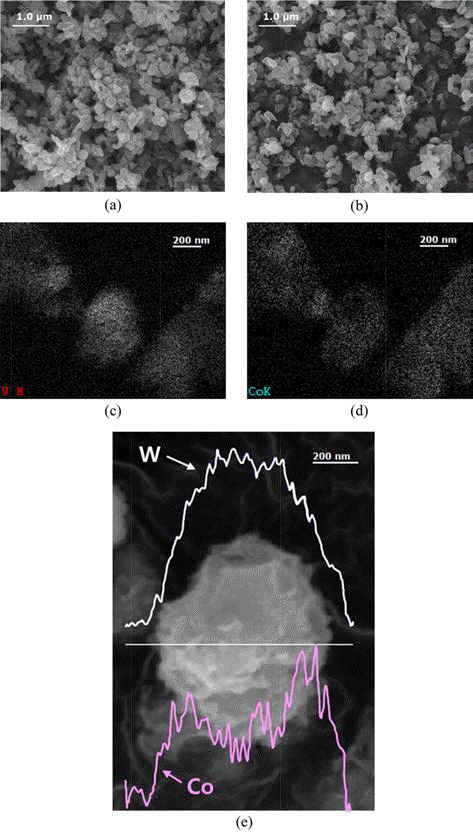

Fig. 5.TEM analyzed result of the WC-Co composite powder, (a) the cross-section of the WC-Co composite powder, (b) WCCo composite powder and interplanar distance and FFT analysis.

- 1. G Jin, B Xu, H Wang, Q Li and S Wei, Mater Lett. (2007) 61 2454.Article
- 2. J Sun, F Zhang and J Shen, Mater Lett. (2003) 57 3140.Article
- 3. O O Eso, P Fan and Z. Z Fang, Int. J. Refract Met. Hard Mater. (2008) 26 91.Article
- 4. H Lin, B Tao, Q Li and Y Li, Mater Res. Bull. (2012) 47 3283.Article
- 5. O Eso, Z Fang and A Griffo, Int. J. Refract Met. Hard Mater. (2005) 23 233.Article
- 6. C Jia, H Tang, X Mei, F Yin and X Qu, Mater Lett. (2005) 59 2566.Article
- 7. H. S Hong, Y. D Ko, L. S Kang, G. H Kim and H. C Jung, J. Kor. Powd. Met. Inst. (2011) 18 244.Article
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Electroless Ni-P deposition on WC powders through direct PdCl2 activation and study on the underlying mechanisms
Peng Tang, Shuwen Jiang, Jiawei Yan, Xianquan Li
Next Materials.2025; 6: 100496. CrossRef - Pre-treatments of initial materials for controlling synthesized TaC characteristics in the SHS process
Jae Jin Sim, Sang Hoon Choi, Ji Hwan Park, Il Kyu Park, Jae Hong Lim, Kyoung Tae Park
journal of Korean Powder Metallurgy Institute.2018; 25(3): 251. CrossRef - Spark plasma sintering of WC–Co tool materials prepared with emphasis on WC core–Co shell structure development
Sungkyu Lee, Hyun Seon Hong, Hyo-Seob Kim, Soon-Jik Hong, Jin-Ho Yoon
International Journal of Refractory Metals and Hard Materials.2015; 53: 41. CrossRef
Coating of Cobalt Over Tungsten Carbide Powder by Wet Chemical Reduction Method


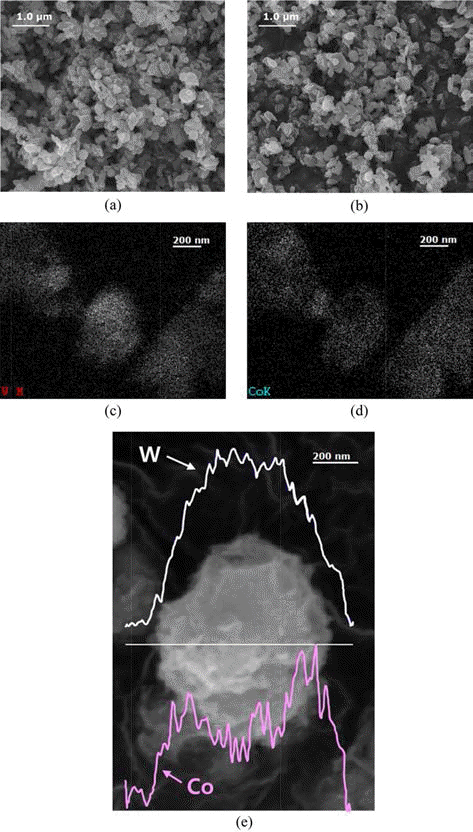
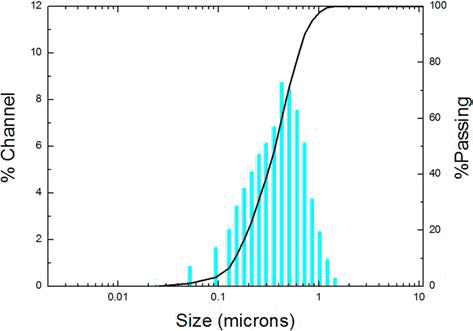
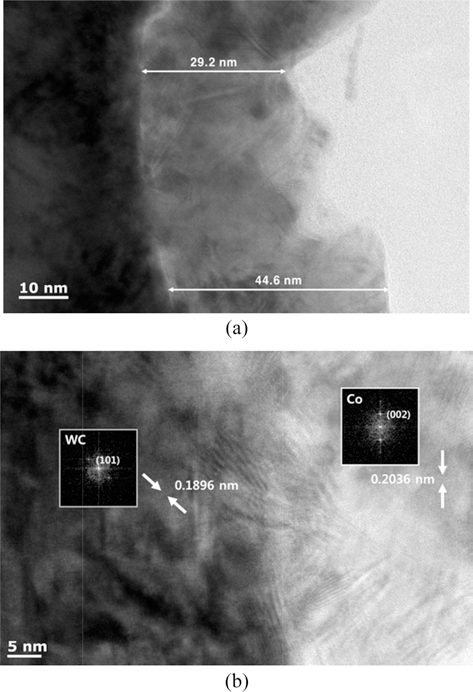
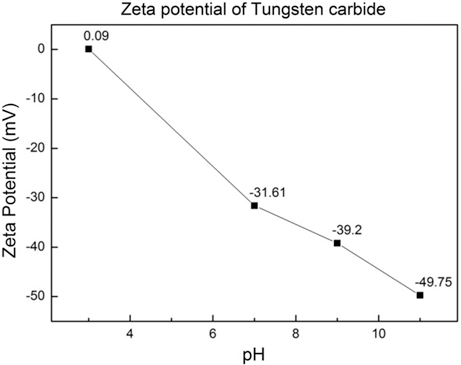
Fig. 1.
Process flow sheet for synthesis of WC-Co composite.
Fig. 2.
The XRD of the WC-Co composite powder.
Fig. 3.
The SEM of the WC-Co composite powder. (a) the pure WC, (b) WC-Co, EDS analysis of (c) W, EDS analysis of (d) Co, and (e) Line scanning analysis.
Fig. 4.
Distribution of WC-Co composite powder.
Fig. 5.
TEM analyzed result of the WC-Co composite powder, (a) the cross-section of the WC-Co composite powder, (b) WCCo composite powder and interplanar distance and FFT analysis.
Fig. 6.
The zeta potential of WC powder at various pH.
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
Coating of Cobalt Over Tungsten Carbide Powder by Wet Chemical Reduction Method
TOP
 KPMI
KPMI

 Cite this Article
Cite this Article






