Articles
- Page Path
- HOME > J Korean Powder Metall Inst > Volume 23(2); 2016 > Article
-
Research Article
- Influences of the Eu Concentration and the Milling Time on Photoluminescence Properties of Y2O3-H3BO3:Eu3+ Powders Prepared by Mechanical Alloying
- Hyun-Sic Gong, Hyun-Goo Kim*
-
Journal of Korean Powder Metallurgy Institute 2016;23(2):108-111.
DOI: https://doi.org/10.4150/KPMI.2016.23.2.108
Published online: March 31, 2016
Department of Physics Education, Chosun University, Gwangju 61452, Korea
- *Corresponding Author: Hyun-Goo Kim, +82-62-230-7376, +82-62-232-8122, hgakim@chosun.ac.kr
• Received: December 30, 2015 • Revised: February 3, 2016 • Accepted: February 25, 2016
© The Korean Powder Metallurgy Institute. All rights reserved
- 615 Views
- 3 Download
Abstract
- Y2O3–H3BO3:Eu3+ powders are synthesized using a mechanical alloying method, and their photoluminescence (PL) properties are investigated through luminescence spectrophotometry. For samples milled for 300 min, some Y2O3 peaks ([222], [440], and [622]) and amorphous formations are observed. The 300-min-milled mixture annealed at 800°C for 1 h with Eu = 8 mol% has the strongest PL intensity at every temperature increase of 100°C (increasing from 700 to 1200°C in 100°C increments). PL peaks of the powder mixture, as excited by a xenon discharge lamp (20 kW) at 240 nm, are detected at approximately 592 nm (orange light, 5Do → 7F1), 613 nm, 628 nm (red light, 5Do → 7F1), and 650 nm. The PL intensity of powder mixtures milled for 120 min is generally lower than that of powder mixtures milled for 300 min under the same conditions. PL peaks due to YBO3 and Y2O3 are observed for 300-min-milled Y2O3–H3BO3 with Eu = 8 mol% after annealing at 800°C for 1 h.
- A luminescent material absorbs various types of energy and emits thermal and electromagnetic radiation in the ultraviolet (UV), infrared, and visible ranges with the last being most common. In particular, inorganic luminescent materials have been studied for more than 100 years and are now used everyday in display technologies, such as fluorescent lamps, cathode ray tubes (CRTs), X-ray plates, advertisement signboards, plasma display panels (PDP), and light emitting diodes (LEDs), as well as in applications in fields such as advanced medicine and security, all of which are areas where people require information.
- Nanocrystalline and amorphous alloys of a desired composition can be synthesized from pure crystalline powder using a mechanical alloying (MA) method [1-4]. Koch [5] applied MA to Ni60Nb40 production, and Schwarz et al. [6] have used it to examine the thermodynamics of amorphous materials.
- Although there have been advancements in luminescent materials since the commercialization of GaN blue LEDs in 1966, such as the development of the white LED, their still requires improvement. Red luminescent materials are not efficient because the difference between their excitation and emission energies is greater than those in the case any other luminescent material. Therefore, highly efficient red luminescent materials, rather than green or blue luminescent materials, for blue LEDs and near-UV LEDs are needed.
- Chong et al. [7] examined the photoluminescent (PL) properties of Y2O3:Eu3+ phosphor films fabricated with a sol-gel process. Dhoble et al. [8] prepared the same by using combustion synthesis and precipitation techniques. PL characteristics and X-ray excitation of films fabricated by using the two different methods were compared. Wang et al. [9] synthesized Y0.95-xMxBO3:0.05Eu3+ (M:Bi3+, La3+, Sc3+, 0 ≤ x ≤ 0.1) for a PDP by using a solid-state reaction and examined it under ultraviolet light. Chen et al. [10] reported the preparation and PL properties of YBO3:Eu3+ powders synthesized by using a solvothermal process with isopropanol used as a solvent.
- This study examined the PL properties of Eu3+-doped Y2O3-H3BO3 powders synthesized by using MA. X-ray diffractometry (XRD) and luminescence spectrophotome- try were used to measure the concentrations of Eu3+ as a function of the annealing time, annealing temperatures, and milling time (tm).
Introduction
- Y2O3-H3BO3:Eu3+ mixtures were prepared by mixing yttrium oxide (Y2O3) and boric acid (H3BO3) at a molar ratio of 1:1, followed by the addition of 1, 2, 4, 6, 8, and 10 mol% europium(III) nitrate hexahydrate (Eu(NO3)3·6H2O) (FW 446.07). The mixtures were then milled using a planetary ball mill at room temperature under the following conditions: 2.68 g powder and 13 zirconia balls, 15 mm in diameter at sample-to-balls weight ratio of 50:1. These were rotated in an 80 mL zirconia jar at 500 rpm. For accurate analysis, a same amount of the mixture was milled under similar conditions, for tm of 0, 15, 30, 60, 120, and 300 min. The grinding operation was stopped for 30 min, which was followed by grinding for 15 min to avoid an excessive increase in temperature inside the mill pot. The as-milled powders were annealed at 700, 800, 900, 1000, 1100, and 1200ºC for 1, 2, and 3 h, respectively.
- XRD (PANalytical, X'pert PRO MPD) was performed at room temperature with Cu Ka (λ = 1.54060 Å) radiation. The acceleration voltage and current were 40 kV and 30 mA, respectively. The irradiation rate was 1 sec/ 0.03 step over the 2θ measurement range of 10-100°. The PL of the Eu doped Y2O3-H3BO3 mixtures was measured between 520 nm and 700 nm as a function of the annealing temperature, annealing time, and tm. A xenon discharge lamp (20 kW) was used under the following conditions: excitation at 240 nm, excitation slit at 10.0 nm, emission slit at 8.0 nm excitation, and scan speeds of 300 nm/min.
Experimental
- Fig. 1 shows the XRD patterns of the Y2O3-H3BO3:Eu3+ mixtures synthesized by MA as a function of tm. The H3BO3 peak at approximately 2θ = 28.0° disappeared in the case of 15 min milling, indicating the formation of a solid solution of H3BO3 in the Y2O3. After milling for 120 min, the XRD intensity began to decrease, and all elemental lines broadened slightly [11]. This peak broadening reflects not only a decrease in crystallite size, but also strain induced by the ball-milling. After milling for 300 min, some Y2O3 peaks and amorphous formations were observed[12].
- Fig. 2 shows the PL intensity of a 300-min-milled Y2O3-H3BO3:Eu3+ (Eu = 8 mol%) powder mixture, milled for 300 min, that had been annealed for 1 h at every temperature increase of 100ºC from 700ºC to 1200ºC, and subjected to 240 nm excitation. The intensity was highest when the mixture was annealed at 800°C, and decreased for annealing temperatures above 800°C. The samples showed a more broad emission, and a shift of the peaks with increasing annealing temperature above 800°C has been recorded. This phenomena of the peaks can be attributed to the changes in the crystal structure [13]. Further investigation on the mechanism of above phenomena and more detailed measurement of Y2O3- H3BO3:Eu3+ system are need. The PL intensity at 613 nm for the mixture of 800°C was highest for the mixture annealed at 800°C, and whereas the intensity at 628 nm was similar regardless of annealing temperature. The overall shape of the luminescence PL intensity differed a little with from that of a Eu = 4 mol% doped mixture [12]. When the mixtures were subjected to 240 nm excitation, the PL intensities were observed at approximately 592 nm, 613 nm, 628 nm, and 650 nm. This might be due to the transition of free Eu3+ ions from the 5Do level to the 7Fj (j = 1-3) level. The orange luminescence peak at approximately 592 nm might be due to the 5Do → 7F1 magnetic dipole transition of the Eu3+ ions. The red luminescence peaks at approximately 613 nm and 628 nm were assigned to the 5Do → 7F2 forced electric-dipole transition [14,15]. The luminescence peak at approximately 650 nm was attributed to a 5Do → 7F3 transition [16]. We compared the PL emission spectra of Y2O3-H3BO3:Eu3+mixture by MA with that of YBO3:Eu3+ samples by electrodeposition and ionic liquid-assisted hydrothermal process [17,18]. While the intensities and the shapes of the PL spectra differed slightly, the peak locations are very similar.
- Fig. 3 shows the variations in PL intensity for Y2O3- H3BO3:Eu3+ powder mixtures due to differing concentrations of Eu and differing annealing temperatures (1 h annealing time). The PL intensity of the powder mixture was highest after annealing at 800°C with the addition of Eu = 8.0 mol% for Y2O3-H3BO3 powder mixture. The PL intensity gradually decreased with increasing annealing temperatures. This phenomenon was different from a Eu = 4.0 mol% doped Y2O3-H3BO3 mixture, with the highest intensity occurring after annealing at 900°C [12].
- Fig. 4 shows the PL intensity of Y2O3-H3BO3:Eu3+ (Eu: 8 mol%) powder mixture milled for 300 min and annealed for 1, 2, and 3 h at 800ºC. Based on the PL intensity dependence annealing time, the highest luminescence intensity was obtained at wavelengths of 613 nm for the 1 h annealed mixture, as it was the case for the mixtures milled for 120 min, regardless of Eu mol%, but the luminescence intensity decreased with increasing annealing time.
- Fig. 5 compares the variation in PL intensities of Y2O3- H3BO3:Eu3+ (Eu:4 mol%, Eu:8 mol%) powder mixtures milled for 120 min and 300 min and then annealed for 1 h at every 100ºC from 700ºC to 1200ºC. The luminescence intensities for powder mixtures milled for 120 min were low, but in general, more than those for powder mixtures milled for 300 min. For the Y2O3-H3BO3:Eu3+ powder mixtures milled for 120 min, the highest PL intensity was observed for annealing temperatures of 1000°C at Eu = 4.0 mol%. The other luminescence intensities of the powder mixture were similar.
- Fig. 6 shows the XRD pattern of the Y2O3-H3BO3:Eu3+ mixture milled for 300 min and annealed at 800ºC for 1 h with Eu = 8 mol%. Peaks corresponding to YBO3 with Y2O3 are observed.
Results and Discussion
Fig. 1
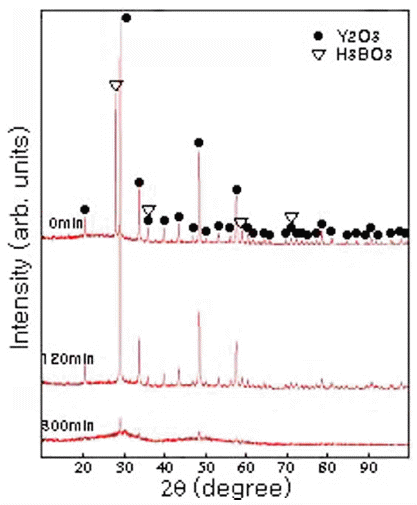
X-ray diffraction patterns of Y2O3-H3BO3:Eu3+ powders fabricated by planetary ball milling as a function of different tm.

Fig. 2
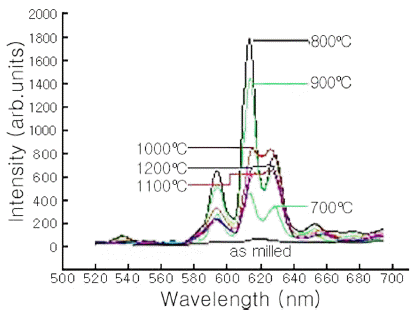
PL emission spectra for Y2O3-H3BO3:Eu3+ (Eu: 8 mol%) mixtures milled for 300 min annealed at the indicated temperatures.

Fig. 3
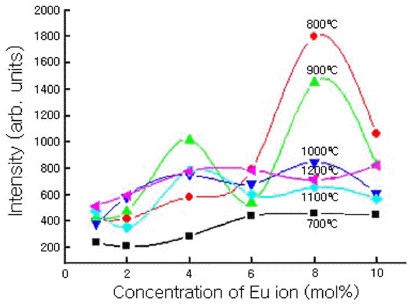
The variations in the PL emission spectra for Y2O3- H3BO3:Eu3+ mixtures (tm = 300 min) as a function of different temperatures and Eu3+concentrations.

Fig. 4
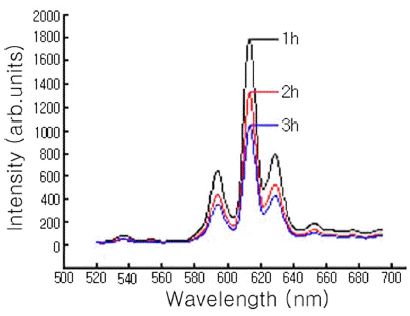
PL emission spectra for Y2O3-H3BO3:Eu3+ (Eu:8 mol%) mixtures milled for 300 min and annealed 800ºC for the indicated time.

Fig. 5
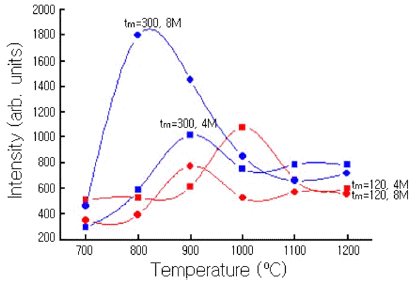
The variations in the PL emission spectra for Y2O3- H3BO3:Eu3+ (Eu:4 mol% and 8 mol%) for milling time of 120 min and 300 min.

- Y2O3-H3BO3:Eu3+ powders were synthesized at room temperature using a MA method. In the case of milling for 300 min, some PL peaks due to Y2O3 ([222], [440], [622]) and amorphous formations were observed. The 300-min-milled mixture that was annealed at 800°C for 1 h with Eu = 8 mol% had the strongest PL intensity for each annealing temperature investigated (increasing from 700ºC to 1200ºC at increments of 100ºC). The PL of the powder mixture, excited by a xenon discharge lamp (20 kW) at 240 nm, was detected at approximately 592 nm (orange color, 5Do → 7F1), 613 nm, 628 nm (red color, 5Do → 7F2), and 650 nm. The highest luminescence intensity was obtained for mixtures annealed for 1 h, but the luminescence intensity decreased with increasing annealing time. The luminescence intensities of powder mixtures milled for 120 min were low in general, but more than those of powder mixtures milled for 300 min, other conditions being equal, therefore we could know that Y2O3- H3BO3:Eu3+ powders must be milled for more than 120 min. The PL peaks due to the presence of YBO3 and Y2O3 were observed after annealing at 800ºC for 1 h for a 300- min-milled Y2O3-H3BO3 with Eu = 8 mol%.
Conclusions
- 1. E Arzt and L Schult, New Materials Mechanical Alloying Techniques, (1988) DGM, Germany 3.
- 2. D Roy, D Chakravarty, R Mitra and I Manna: J. Alloys Compd., (2008) 460 320.Article
- 3. S Kumaran, T Sasikumar, R Arockiakumar and T S Rao: Powder Technol., (2008) 185 124.Article
- 4. H G Kim and W N Myung: Inter. J. Non-Equilibrium Processing., (1998) 10 305.Article
- 5. C C Koch, O B Cavin, C G McKamey and J O Scarbrough: Appl. Phys. Lett., (1983) 43 1017.ArticlePDF
- 6. R B Schwartz, R R Petrich and C K Saw: J. Non- Cryst. Solids., (1985) 76 281.
- 7. M K Chong, K Pita and C H Kam: J. Phys. Chem. Solids., (2005) 66 213.Article
- 8. S J Dhoble, I M Nagpure, J G Mahakhode, S V Godbole, M K Bhide and S V Moharil: Nucl. Instrum. Methods Phys. Res. B., (2008) 266 3437.Article
- 9. Y Wang and L Wang: Mater. Lett., (2006) 60 2645.Article
- 10. F-S Chen, C-H Hsu and C-H Lu: J. Alloys Compd., (2010) 505 L1.Article
- 11. H S Gong, M.S. Thesis, Photoluminescence of Y2O3-H3BO3:Eu3+ Powders by Mechanical Alloying. (2011) Chosun University, Gwangju 30.
- 12. H-S Gong and H-G Kim: Curr. Appl. Phys., (2013) 13 453.
- 13. A Yousif, R M Jafer, S Som, M M Duvenhage, E Coetsee and H C Swart: Appl. Surf. Sci., (2016) 365 93.Article
- 14. X Guo, Y Wang and J J Zhang: Cryst. Growth., (2009) 311 2409.Article
- 15. L Muresan, E J Popovici, F I Lucaci, R Grecu and E Indrea: J. Alloys Compd., (2009) 483 346.Article
- 16. J Zhang and J Lin: J. Cryst. Growth., (2004) 271 207.Article
- 17. W Pan, P Wang, Y Xu and R Liu: Thin Solid Film., (2015) 578 69.Article
- 18. Y Tian, B Tian, B Chen, C Cui, P Huang, L Wang and R Hua: J. Alloys Compd., (2014) 590 61.Article
Reference
Figure & Data
References
Citations
Citations to this article as recorded by 

Influences of the Eu Concentration and the Milling Time on Photoluminescence Properties of Y2O3-H3BO3:Eu3+ Powders Prepared by Mechanical Alloying





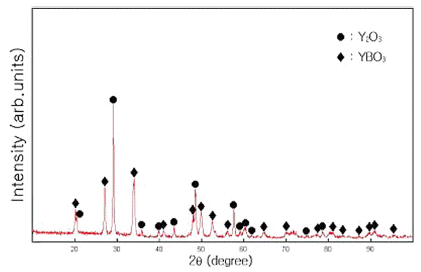
Fig. 1
X-ray diffraction patterns of Y2O3-H3BO3:Eu3+ powders fabricated by planetary ball milling as a function of different tm.
Fig. 2
PL emission spectra for Y2O3-H3BO3:Eu3+ (Eu: 8 mol%) mixtures milled for 300 min annealed at the indicated temperatures.
Fig. 3
The variations in the PL emission spectra for Y2O3- H3BO3:Eu3+ mixtures (tm = 300 min) as a function of different temperatures and Eu3+concentrations.
Fig. 4
PL emission spectra for Y2O3-H3BO3:Eu3+ (Eu:8 mol%) mixtures milled for 300 min and annealed 800ºC for the indicated time.
Fig. 5
The variations in the PL emission spectra for Y2O3- H3BO3:Eu3+ (Eu:4 mol% and 8 mol%) for milling time of 120 min and 300 min.
Fig. 6
X-ray diffraction patterns for Y2O3-H3BO3:Eu3+ (Eu: 8 mol%) mixtures annealed at 800ºC for 1 h.
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
Fig. 6
Influences of the Eu Concentration and the Milling Time on Photoluminescence Properties of Y2O3-H3BO3:Eu3+ Powders Prepared by Mechanical Alloying
TOP
 KPMI
KPMI


 Cite this Article
Cite this Article






