Articles
- Page Path
- HOME > J Korean Powder Metall Inst > Volume 23(2); 2016 > Article
-
Research Article
-
Effects of Hydrogen Reduction in Microstructure, Mechanical and Thermoelectric Properties of Gas Atomized
n -type Bi2Te2.7 Se0.3 Material - Pradip Rimal, Sang-Min Yoon, Eun-Bin Kim, Chul-Hee Lee, Soon-Jik Hong*
-
Journal of Korean Powder Metallurgy Institute 2016;23(2):126-131.
DOI: https://doi.org/10.4150/KPMI.2016.23.2.126
Published online: March 31, 2016
Division of Advanced Materials Engineering, Kongju National University 32588, Korea
- *Corresponding Author: Soon-Jik Hong, +82-41-521-9387, +82-41-568-5776, hongsj@kongju.ac.kr
• Received: April 4, 2016 • Revised: April 15, 2016 • Accepted: April 20, 2016
© The Korean Powder Metallurgy Institute. All rights reserved
- 936 Views
- 5 Download
- 6 Crossref
Abstract
- The recent rise in applications of thermoelectric materials has attracted interest in studies toward the fabrication of thermoelectric materials using mass production techniques. In this study, we successfully fabricate n-type Bi2Te2.7Se0.3 material by a combination of mass production powder metallurgy techniques, gas atomization, and spark plasma sintering. In addition, to examine the effects of hydrogen reduction in the microstructure, the thermoelectric and mechanical properties are measured and analyzed. Here, almost 60% of the oxygen content of the powder are eliminated after hydrogen reduction for 4 h at 360°C. Micrographs of the powder show that the reduced powder had a comparatively clean surface and larger grain sizes than unreduced powder. The density of the consolidated bulk using as-atomized powder and reduced atomized powder exceeds 99%. The thermoelectric power factor of the sample prepared by reduction of powder is 20% better than that of the sample prepared using unreduced powder.
- Thermoelectric (TE) materials directly convert heat energy to electricity i.e. when a temperature gradient is provided on thermoelectric junction a voltage gradient is produced through the Seebeck effect [1]. Waste heat from the automotive exhaust, home heating, and industrial processes are significant and recovery of these wasted heat with thermoelectric generators optimizes sustainability of energy resources [2]. Salient features like high reliability, absence of moving parts, soundless operation and low environmental impact have attracted researchers to develop efficient material for more than six decades [3]. Efficiency of TE materials can be estimated by unit less figure of merit [4,5],
- where, σ is conductivity; S is seebeck coefficient; T is temperature expressed in Kelvin and κ is thermal conductivity; of the material. Though significant increment in individual properties of S, σ and κ are reported, their interrelated factors and coupling between them have limited ZT value around 1. Solid state system exhibiting ZT ≥ 3 is expected to supersede conventional mechanical energy conversion devices [6].
- Rhombohedral crystal structure of Bi2(Te,Se)3 has a five layered units of Bi and (Te,Se) hexagonal planes stacked along the base plane, c-axis with atomic order (Te,Se)-Bi-(Te,Se)-Bi-(Te,Se) or Te-Bi-Se-Bi-Te [7, 8]. The n-type Bi2Te3-Bi2Se3 compound has exhibited outstanding TE properties near room temperature. However, full functionality of n-type materials is limited by oxidation on powder surface, which acts as donor thereby degrading the thermoelectric performance of these materials. Appropriate selection of hydrogen reduction treatment condition can reduce oxidation amount to near solubility level of oxygen in Bi2(Te,Se)3 based materials [9].
- TE materials prepared by directional solidification method like zone melting possess outstanding electrical properties, but their applications are limited by poor mechanical properties since their basal plane is a cleavage plane [10,11]. At present, gas atomization process is welladapted powder fabrication technique for mass production and high performance spherical shaped powders [12]. Powder as produced by gas atomization are more homogeneous and has finer grained structures and also reduces the complications required in melting and solidification processing [13]. Spark Plasma Sintering (SPS) is a fascinating technology to fabricate fine grains and highdensity TE materials. Controlled grain growth, less waste and shorter time of sintering make it most promising consolidation technique for mass production [14].
- In this study, the effects of hydrogen reduction in microstructure, mechanical and thermoelectric properties of n-type Bi2Te2.7Se0.3 material fabricated by gas atomization and spark plasma sintering are studied.
Introduction
- 2.1 Powder fabrication and consolidation
- Pellets of elemental Bi, Te, and Se ingots of high purity > 99.99% were mixed and heated inside graphite crucible to obtain the melt of Bi2Te2.7Se0.3 alloy by using highfrequency induction furnace. The as formed alloy melt was then introduced through a boron nitride melt delivery nozzle into a gas atomizer operating under argon atmosphere. The pressure of inert atomization gas was fixed at 1.2 MPa until atomization of all melt was assured. Thus, Bi2Te2.7 Se0.3 alloy powder of wide size distribution was obtained and subsequently sieved so that powder and satellites of size above 200 μm were excluded from further analysis. Some portion of Gas atomized (GA) powder was followed by hydrogen reduction treatment in Electric Tube Furnace to eliminate the oxygen present in the powder. The heating rate of the furnace was 10°C/min for 36 min and stabilized at reduction temperature 360°C for 4 hours before cooling to room temperature. The reduced gas atomized (RGA) powder was kept in a protective environment of Glove Box avoiding any chances of oxidation. In this study, both types of powder GA and R-GA were consolidated by Spark plasma sintering technique. Graphite mold filled with 15 g of powder was sintered in vacuum at 400°C temperature for 10 min under a uniaxial pressure of 50 MPa.
- 2.2 Microstructure and phase analysis
- Powder surface morphology of both GA and R-GA powder was analyzed by field emission scanning electron microscopy (FESEM). The specimen for powder microstructure observation was mounted using epoxy resin and the surface was polished using suitable grades of sand papers and alumina paste. The microstructures of both GA and R-GA powder were observed through an optical microscope. Phase identifications of powder and bulks were investigated by X-Ray Diffraction (Mini- Flex-600; Rigaku, Japan) using CuK-beta(α2) radiation. To investigate the bulk microstructures of samples consolidated by SPS, both GA and R-GA samples were fractured in the direction perpendicular to the press direction and FESEM images of the fractured surfaces were studied.
- 2.3 Physical and mechanical properties
- Oxygen contents of GA and R-GA powder were measured by Eltra ONH-2000 Oxygen/Nitrogen/Hydrogen determinator. The relative density of the bulk samples were measured for ten times at ambient temperature using Archimedes Principle, and average results are reported. A polished sample mounted in epoxy resin was used to measure the hardness of both GA and RGA sample. Vickers hardness of samples was measured at different locations of mounted sample piece by hardness tester (MMT-X3; Japan) for 20 times under the load of 98.07×10−3 kgf and averaged; the average values were reported as hardness result and used for further analysis.
- 2.4 Thermoelectric properties
- TE properties of both GA and R-GA bulk samples were measured in a direction normal to the sintering pressure direction. Samples of size 3 mm × 3 mm × 10 mm were cut from disc bulk and the cut samples were polished and cleaned to measure TE transport properties. Temperature dependence of seebeck coefficient, electrical conductivity and power factors of the samples were measured by Thermoelectric Power measurement system (TEP 1000; South Korea) near room temperature. Carrier concentration (η) and Hall mobility (μ) were measured on samples of size 5 mm × 5 mm × 0.5 mm at room temperature.
Experimental Procedure
- 3.1 Microstructure and Phase identification
- Powder fabrication method has a direct influence on shape, size, and surface of prepared powder. Powder morphology and powder microstructure dictate mechanical and TE properties of Bi2Te3 based materials. In this study, morphology and microstructure of powder used for sintering were investigated using field emission scanning electron microscope and optical microscope. Fig. 1 shows the SEM and OM images of the GA and R-GA powder. Most of the powder had near spherical shapes and fine surface. Fig. 1(a) shows the powder morphology of gas atomized Bi2Te2.7Se0.3 material. Most of the GA powder had near spherical shape, which is very attractive for mold filling during sample fabrication as spherical powders exhibit better flowability and higher tap density compared to other shapes. Typical to the GA process, we can notice that GA powder shows a broad range of size distribution. Generally GA powder size ranges from submicron to 200 μm. Further, high gas jet pressure and rapid solidification during atomization convert small amount of melt into dust particles. Fig. 1(b) shows the powder morphology of R-GA powder. Also, it is necessary to mention that hydrogen reduction in this study does not show any notable effect in powder shape and size distribution, but the process significantly influenced powder microstructure. Fig. 1(c) shows the microstructure of the GA powder. Needle-shaped grains of different sizes are clearly visible on the microstructure of GA powder. To study the effect of reduction in the microstructure, reduced powder of similar size was selected and compared, as grain sizes are dependent on powder sizes in the atomized powder. The difference in powder microstructure can be easily noticed in Fig 1(d). As expected after reduction, grain sizes of the powder increased which suggests that R-GA powder had larger grain sizes and less number of grains.
- Fig. 2 shows the XRD patterns of the powder and SPS bulks. All powder and bulks followed Standard powder diffraction JCPDS index Bi2Te3 (PDF# 015-0863) peaks suggesting powder and bulks consisted single phase rhombohedral structure. Although reduction was carried out at a higher temperature for a long time, reduced powder also followed the same diffraction pattern, which indicates that powder phase and structure were intact without any compositional change. N-type Bi2Te3 based materials are anisotropic in nature and orientation of caxis of grains along <00l> plane is useful to yield high TE properties [8]. However, cleavage plane lies parallel to c-axis in Bi2Te3 based material so strong orientations deteriorates mechanical properties and makes it less desirable for practical applications [11,15]. As seen in Fig. 2, the XRD intensities along basal planes (006) and (0015) were weak for both GA and R-GA powder and corresponding consolidated samples. This signifies that SPS consolidation does not stimulate any preferential orientation in consolidated samples and in this study both GA and R-GA bulk samples had isotropic mechanical and TE properties.
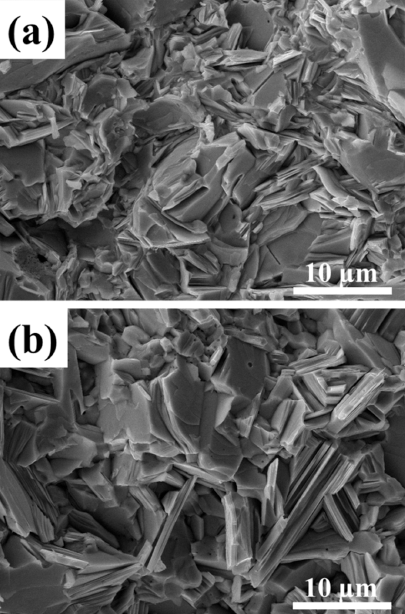
- Microstructures of bulk samples provide useful information regarding the behavior of TE material and their performance. Fig. 3 shows the fracture surfaces of the consolidated bulk samples. Grains of different sizes are visible in the SEM images. Also, no voids or pores could be located. Smaller grain sizes and a large number of grain boundaries are mostly desirable in TE materials for the reduction of thermal conductivity. Grain sizes in Fig. 3(b) are larger in comparison to those in Fig. 3(a) which could be attributed to the larger grain sizes of the reduced powder. Also, no special grain orientations were seen in the fractographs which support the result obtained from XRD analysis that samples were isotropic in nature.
- 3.2 Physical parameters and mechanical properties
- Gas atomization though acclaimed for minor oxidation of fabricated powder than existing other powder fabrication techniques, nullifying the oxidation of powder is out of hand. The oxygen content of the GA powder, 402.2 ppm, was significantly reduced to 180.6 ppm in R-GA powder through hydrogen reduction. Despite the drastic elimination of oxygen from powder after reduction, considerable amount of residual oxygen present in powder was inherited as the solubility of oxygen in Bi2(Te,Se)3 [9]. The density of bulk samples also has a decisive role in the performance of TE material. Higher densities are required to ensure less material loss during sample fabrication and to yield optimum TE properties. Density of bulk samples is dependent on the powder morphology, size distribution and consolidation process. In this study, use of wide size distribution spherical powder and optimized condition of SPS consolidation of Bi2Te3 based materials resulted in densities above 99% for both GA and R-GA samples. Both GA and R-GA bulk samples possessed enough strength for a practical application, which was practically realized during sample preparation. Vickers hardness was around 60 for both GA and RGA bulk samples. Though slight grain growth was observed after reduction, it is interesting that mechanical property of as-fabricated samples was not influenced by the hydrogen reduction treatment.
- 3.3 Thermoelectric properties
- Fig. 4 shows the temperature dependence of electrical conductivity of the samples consolidated by SPS measured at room temperature range of 25-50°C. Electrical conductivity can be expressed by following equation [16]:
- Where, η is the carrier concentration, e is charge carried by an electron and μc is carrier mobility in the low carrier concentration limit [1]. From Eq. (2), it can be seen that electrical conductivity depends on the product of carrier concentration and carrier mobility. High electrical conductivity was measured in GA bulk sample, but after reduction electrical conductivity of the R-GA bulk sample plummeted. In this study, a decrease in electrical conductivity after reduction was mostly influenced by an enormous loss in carrier concentration although there was a slight increment in carrier mobility as shown in Fig. 5.
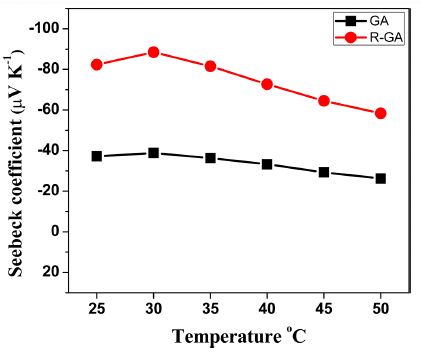
- Seebeck coefficient of all the samples measured at a temperature range of 25-50°C is plotted in Fig. 6. Seebeck coefficient determines the difference in voltage generated for temperature difference created at two ends of thermoelectric material. Seebeck coefficient is simply expressed as [1]:
- Where, ΔV is difference in thermoelectric voltage measured at terminals of samples and ΔT is temperature difference created at the terminals. In our study, negative values obtained for both samples confirmed that they were n-type thermoelectric materials. Seebeck coefficient increased noticeably more than twice for R-GA sample than GA sample. Dissolved oxygen in Bi2Te3- Bi2Se3 solid solutions takes in place of Te(2) which has the smallest electronegativity. This leads to the formation of Bi2Te3-xOx, a solid solution of Bi2Te3 and Bi2O3, in which the dissolved oxygen acts as a donor [9,17]. This explains the higher carrier concentration of GA sample and the significant decrease in carrier concentration after reduction. Also, Seebeck coefficient can be related to career concentration and scattering by following relation [18]:
- Where r is the scattering factor and η is the carrier concentration. Although the presence of oxides could add to scattering of electrons, Seebeck coefficient in this study was mostly influenced by changes in carrier concentration after reduction. In addition, removal of dissolved oxygen can lead to the creation of vacancies at Bi and Te/Se sites, and the created vacancy defects have potential to increase scattering of electrons.
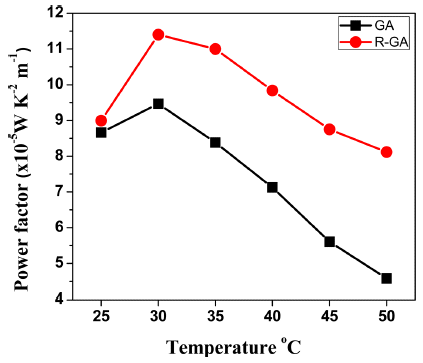
- Worthiness of a material to be classified as TE material relies mostly on the amount of power factor generated by the material, but it is not the only feature to influence performance of TE material. Power Factor of TE materials is defined as [19]:
- Where, σ is the electrical conductivity and S is Seebeck coefficient of the material. Temperature dependence of thermoelectric power factor is shown in Fig. 7. Very low Seebeck coefficient of GA bulk yielded low power factor compared to R-GA bulk sample. For both GA and R-GA bulks, power factor first increased with increase in temperature and peaked at 30°C and then decreased with further increase of temperature. Also, compared to R-GA bulk, power factor of GA bulk sharply declined above 30°C. Almost 20% increment in peak Power Factor and more stability in higher temperature were obtained for R-GA suggesting that hydrogen reduction process can successfully enhance TE properties of n-type Bi2Te3 based materials fabricated by gas atomization process.
Results and Discussions
Fig. 1
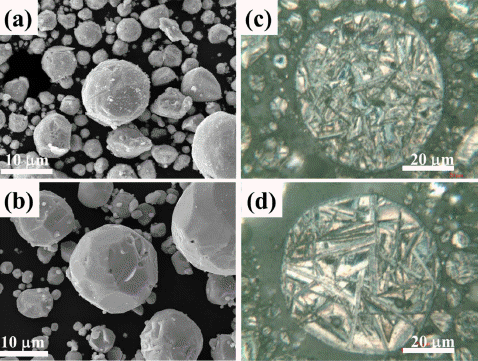
Morphology and microstructure of gas atomized powder: (a) Secondary electron images showing morphology of GA powder (b) Optical microscopy image of microstructure of GA powder (c) Secondary electron images of R-GA powder (d) Optical microscopy image of microstructure of R-GA powder.

Fig. 2
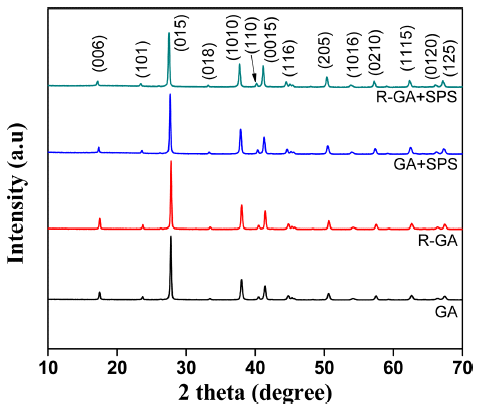
XRD patterns of the GA and R-GA powder and corresponding SPS consolidated bulk samples. Both powder and bulk samples can be indexed to JCPDS (PDF # 015- 0863).

Fig. 5

Carrier concentration η and carrier mobility μc of the samples measured at the room temperature.

- We have presented hydrogen reduction method as an appropriate technique to improve the TE performance of Bi2Te3 based materials fabricated by gas atomization without any effect on mechanical properties. After reduction, we obtained smooth dust free powder surface and almost 60% of oxygen content in the powder was eliminated. Powder microstructure and bulk fracture surface morphology confirmed enlargement in grain size after reduction, but reduction had no effect on Bi2Te3 phase and mechanical properties of SPS consolidated bulk samples. In thermoelectric properties, hydrogen reduction sharply decreased electrical conductivity whereas Seebeck coefficient increased more than twice than that of the sample prepared from unreduced powder. Reduction method had positive effects in improving the thermoelectric power factor by 20%.
Conclusions
-
Acknowledgements
- This work was supported by ‘Energy Efficiency & Resources Core Technology Program’ of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) granted financial resource from the Ministry of Trade, Industry & Energy, Republic of Korea (20152020001210).
Acknowledgements
- 1. D M Rowe, CRC Handbook of Thermoelec, (1995) CRC Press LLC, Boca Raton.
- 2. G J Snyder and E S Toberer: Nat. Mater., (2008) 7 105.ArticlePDF
- 3. Y Lan, A J Minnich, G Chen and Z Ren: Adv. Funct. Mater., (2010) 20 357.Article
- 4. L D Zhao, B-P Zhang, W S Liu, H L Zhang and J-F Li: J. Alloys Compd., (2009) 467 91.Article
- 5. K T Kim, I Son and G H Ha: J. Korean Powder Metall. Inst., (2013) 20 345.Article
- 6. C J Vineis, A Shakouri, A Majumdar and M G Kanatzidis: Adv. Mater., (2010) 22 3970.Article
- 7. A Hruban, A Materna, W Dalecki, G Strzelecka, M Piersa, E J Wegner, R Diduszko, M Romaniec and W Orowski: Acta Phys. Pol. A., (2011) 120 950.Article
- 8. J Jiang, L Chen, S Bai, Q Yao and Q Wang: Mater. Sci. Eng. B., (2005) 117 334.Article
- 9. C H Lim, D C Cho, Y S Lee and C H Lee: J. Korean Phys. Soc., (2005) 46 995.
- 10. H P Ha, Y J Oh, D B Hyun and E P Yoon: Int. J. Soc. Mater. Eng. Resour., (2002) 10 130.Article
- 11. C-H Kuo, C-S Hwang, M-S Jeng, W-S Su, Y-W Chou and J-R J Ku: Alloys Compd., (2010) 496 687.Article
- 12. H-S Kim and S-J Hong: J. Alloys Compd., (2014) 586 S428.Article
- 13. S-J Hong and B-S Chun: Mater. Res. Bull., (2003) 38 599.Article
- 14. L D Zhao, B-P Zhang, J-F Li, M Zhou and W S Liu: Physica B Condens. Matter., (2007) 400 11.Article
- 15. D H Kim, C Kim, S H Heo and H Kim: Acta Mater., (2011) 59 405.Article
- 16. S-J Hong, Y-S Lee, J-W Byeon and B-S Chun: J. Alloys Compd., (2006) 414 146.Article
- 17. F Li, X Huang, Z Sun, J Ding, J Jiang, W Jiang and L Chen: J. Alloys Compd., (2011) 509 4769.Article
- 18. C-H Lee, M F Kilicaslan, B Madavali and S-J Hong: Res. Chem. Intermed., (2014) 40 2543.ArticlePDF
- 19. S-J Hong and B-S Chun: Mater. Sci. Eng. A., (2003) 356 345.Article
Reference
Figure & Data
References
Citations
Citations to this article as recorded by 

- Tuning of power factor in bismuth selenide through Sn/Te co doping for low temperature thermoelectric applications
Ganesh Shridhar Hegde, Ashwatha Narayana Prabhu, Ramakrishna Nayak, C. F. Yang, Y. K. Kuo
Applied Physics A.2024;[Epub] CrossRef - Enhancing thermoelectric performance of K-doped polycrystalline SnSe through band engineering tuning and hydrogen reduction
Nan Xin, Yifei Li, Guihua Tang, Longyun Shen
Journal of Alloys and Compounds.2022; 899: 163358. CrossRef - The effect of powder pre-treatment on the mechanical and thermoelectric properties of spark plasma sintered N-type bismuth telluride
Ahmed A. Abdelnabi, Vickram Lakhian, Joseph R. McDermid, James S. Cotton
Journal of Alloys and Compounds.2021; 874: 159782. CrossRef - Investigation of Spark Plasma Sintering Temperature on Microstructure and Thermoelectric Properties of p-type Bi-Sb-Te alloys
Jin-Koo Han, Dong-won Shin, Babu Madavali, Soon-Jik Hong
Journal of Korean Powder Metallurgy Institute.2017; 24(2): 115. CrossRef - The Preparation and Growth Mechanism of the Recovered Bi2Te3 Particles with Respect to Surfactants
Hyeongsub So, Eunpil Song, Yong-Ho Choa, Kun-Jae Lee
Journal of Korean Powder Metallurgy Institute.2017; 24(2): 141. CrossRef - Enhanced thermoelectric cooling properties of Bi2Te3−xSex alloys fabricated by combining casting, milling and spark plasma sintering
Seung Tek Han, Pradip Rimal, Chul Hee Lee, Hyo-Seob Kim, Yongho Sohn, Soon-Jik Hong
Intermetallics.2016; 78: 42. CrossRef
Effects of Hydrogen Reduction in Microstructure, Mechanical and Thermoelectric Properties of Gas Atomized n -type Bi2Te2.7 Se0.3 Material



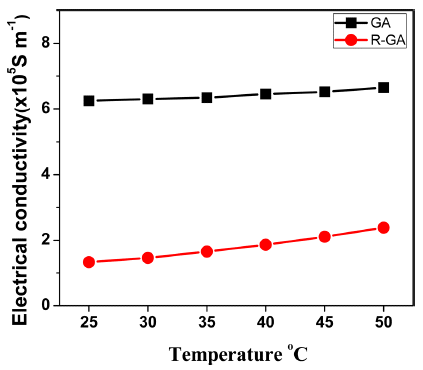
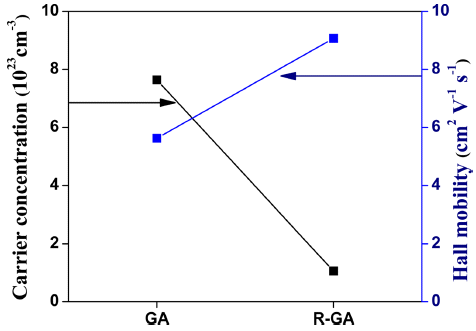
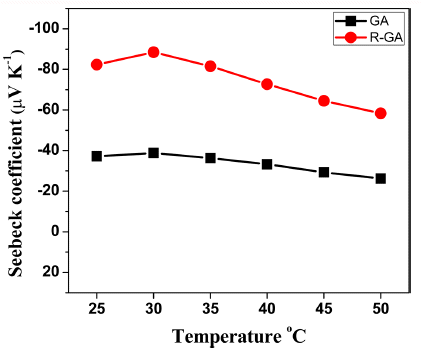

Fig. 1
Morphology and microstructure of gas atomized powder: (a) Secondary electron images showing morphology of GA powder (b) Optical microscopy image of microstructure of GA powder (c) Secondary electron images of R-GA powder (d) Optical microscopy image of microstructure of R-GA powder.
Fig. 2
XRD patterns of the GA and R-GA powder and corresponding SPS consolidated bulk samples. Both powder and bulk samples can be indexed to JCPDS (PDF # 015- 0863).
Fig. 3
Fractographs of SPS consolidated bulk samples: (a) GA sample (b) R-GA sample.
Fig. 4
Temperature dependence of electrical conductivity σ of GA and R-GA sample.
Fig. 5
Carrier concentration η and carrier mobility μc of the samples measured at the room temperature.
Fig. 6
Temperature dependence of Seebeck coefficient S of GA and R-GA samples.
Fig. 7
Temperature dependence of Power Factor PF of GA and R-GA samples measured in temperature range of 25- 50°C.
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
Fig. 6
Fig. 7
Effects of Hydrogen Reduction in Microstructure, Mechanical and Thermoelectric Properties of Gas Atomized n -type Bi2Te2.7 Se0.3 Material
TOP
 KPMI
KPMI


 Cite this Article
Cite this Article







