Articles
- Page Path
- HOME > J Korean Powder Metall Inst > Volume 25(3); 2018 > Article
-
ARTICLE
- Preparation of the MnO2/Macroporous Carbon for PET Glycolysis
- Bong Gill Choia, MinHo Yangb,*
-
Journal of Korean Powder Metallurgy Institute 2018;25(3):203-207.
DOI: https://doi.org/10.4150/KPMI.2017.25.3.203
Published online: May 31, 2018
a Department of Chemical Engineering, Kangwon National University, Samcheok 25913, Republic of Korea
b Department of Energy Engineering, Dankook University, Cheonan 31116, Republic of Korea
- *Corresponding Author: MinHo Yang, TEL: +82-41-550-3685, FAX: +82-41-559-7914, mhyang@dankook.ac.kr
• Received: May 31, 2018 • Revised: June 7, 2018 • Accepted: June 11, 2018
© The Korean Powder Metallurgy Institute. All rights reserved.
- 619 Views
- 3 Download
Abstract
- Plastic pollution is threatening human health and ecosystems, resulting in one of the biggest challenges that humanity has ever faced. Therefore, this study focuses on the preparation of macroporous carbon from biowaste (MC)-supported manganese oxide (MnO2) as an efficient, reusable, and robust catalyst for the recycling of poly(ethylene terephthalate) (PET) waste. As-prepared MnO2/MC composites have a hierarchical pore network and a large surface area (376.16 m2/g) with a narrow size distribution. MnO2/MC shows a maximum yield (98%) of bis(2-hydroxyethyl)terephthalate (BHET) after glycolysis reaction for 120 min. Furthermore, MnO2/MC can be reused at least nine times with a negligible decrease in BHET yield. Based on this remarkable catalytic performance, we expect that MnO2-based heterogeneous catalysts have the potential to be introduced into the PET recycling industry.
- Since human produced the plastics at large-scale in the early 1950s, it has began to immensely spread into our daily life and industral area due to low cost, light weight, high resistance to water, impact, and corrosion, excellent durability, and easy of manufacture [1,2]. Up to now, more than 8.3 billion tons of the plastics have been produced, then discarded 55.4% and recycled only 9% after single use, giving rise to the plastic pollution [1]. The accumulated plastic waste currently poses a threat to land and ocean ecosystem because of a harmful effect on animals and a latent toxicity [3,4]. Therefore, the recylcing technology of plastic waste needs to suppress the plastic pollution that threaten human health and environment.
- Two different techincal routes have been exploited to convert the plastics into fuel and higher-value products: thermolysis and chemolysis [5]. Glycolysis is one of the most studied chemical processes to recover the monomers or small fragments of polymers from thermoplastics, such as poly(ethylene terephtalate) (PET), polyurethane (PU), polycarbonates (PC), and etc [6-8]. For instance, the PET is depolymerized to bishydroxyethylene terephthalate (BHET) monomer with or without the trans-esterification catalysts [9]. Catalytic glycolysis is the most popular due to the sluggish reaction kinetics in the absence of catalysts [10]. During the last decade, numerous catalysts, such as metal acetates, metal oxides, solid superacids, alkalies, sulphates, and ionic liquids have been shown to be effective to improve the reaction rate and efficiency of the PET glycolysis [9,11]. However, these catalyts suffer from several drawbacks involving need to high expense, severe reaction conditions, poor selectivity of the BHET, difficulity in catalyst regeneration, and residual impurity. For this reason, it is highly desirable to develop an efficient, cost-effective, and easily separable catalyst.
- In this work, we demonstated the synthesis of stable MnO2-based heterogenous catalyts for PET recycling process. The macroporous carbons (MCs) from biowaste were used as a catalytic support owing to well-defined pore systems, tunable functionality, inertness, rigidity at high termpertures, cost effectiveness, relatively high surface area, and mass availability [12]. Vertically-oriented MnO2 nanosheets were directly growth on MCs via hydrothermal reaction. The resulting MnO2/MC composites showed 3D-reactant accessible surface, large amount of reactive site for transesterification, leading to enhanced catalytic activity toward PET decomposition.
Introduction
- A hydrothermal method was used to prepare the MnO2/ MC composites. Briefly, the MCs (10 mg) was mixed with the solution (0.1 M KMnO4 : 1 M MnSO4·xH2O, 1:1 v/v) and then poured into a Teflon-lined autoclave. The autoclave was left in the oven heated to 14°oC for 3 hr. The MnO2/MC samples were finally obtained after filtration, washing, and drying process. The resulting samples were characterized using scanning electron microscopy (SEM, S-4800, Hitachi), X-ray diffraction (XRD, Rigaku D/MAX-2500), X-ray photoelectron spectroscopy (XPS, Thermo MultiLab 2000), and Brunauer−Emmett−Teller (BET) analysis (Micrometrics ASAP 2020). For PEG glycolysis, PET resin pellets (Huvis, South Korea) were used after milling. PET powder, anhydrous ethylene glycol (EG) and the 1 wt% MnO2/MC (PET:EG ratio of 10:1) were gently mixed for 15 min and then poured into a reactor. The reaction was performed at 200oC for 30, 60, 90, and 120 min. After glycolysis of PET, the product was evaluated using highperformance liquid chromatography (HPLC, Agilent HPLC 1000 series with Zorbzx C8 column) and nuclear magnetic resonance (NMR, Agilent 400 MHz, 54 mm NMR DD2 spectrometer). The yield of BHET (mol%) is defined as [13]
Experimental
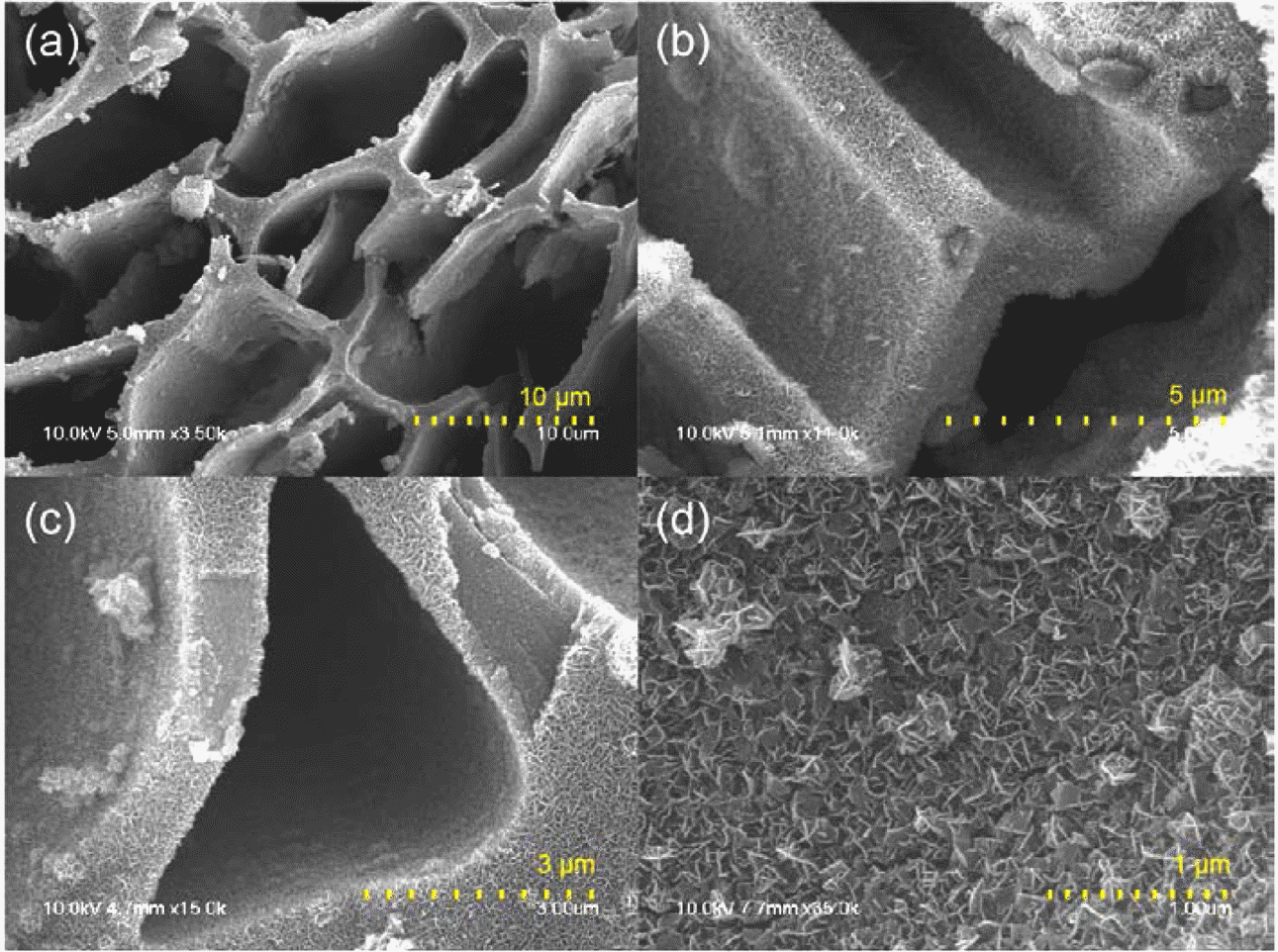
- The morphology of as-synthesized MnO2/MC composites was observed by SEM. As shown in Figure 1(a) and 1(b), MCs were entirely covered with MnO2 nanosheets by the hydrothermal reaction, indicating that MnO2 nanosheets were grown on all the inside wall of MCs. The tubular shape of MCs might enable the precursor molecules to enter easily inside the pores, resulting in totally deposition of MnO2. It is also observed that the original porous structure of the MCs maintained after 3 hr growth reactions. The thickness of MnO2 layer is around 300 nm with the interconnected porous structures on carbon surface (Figure 1(c) and 1(d)).
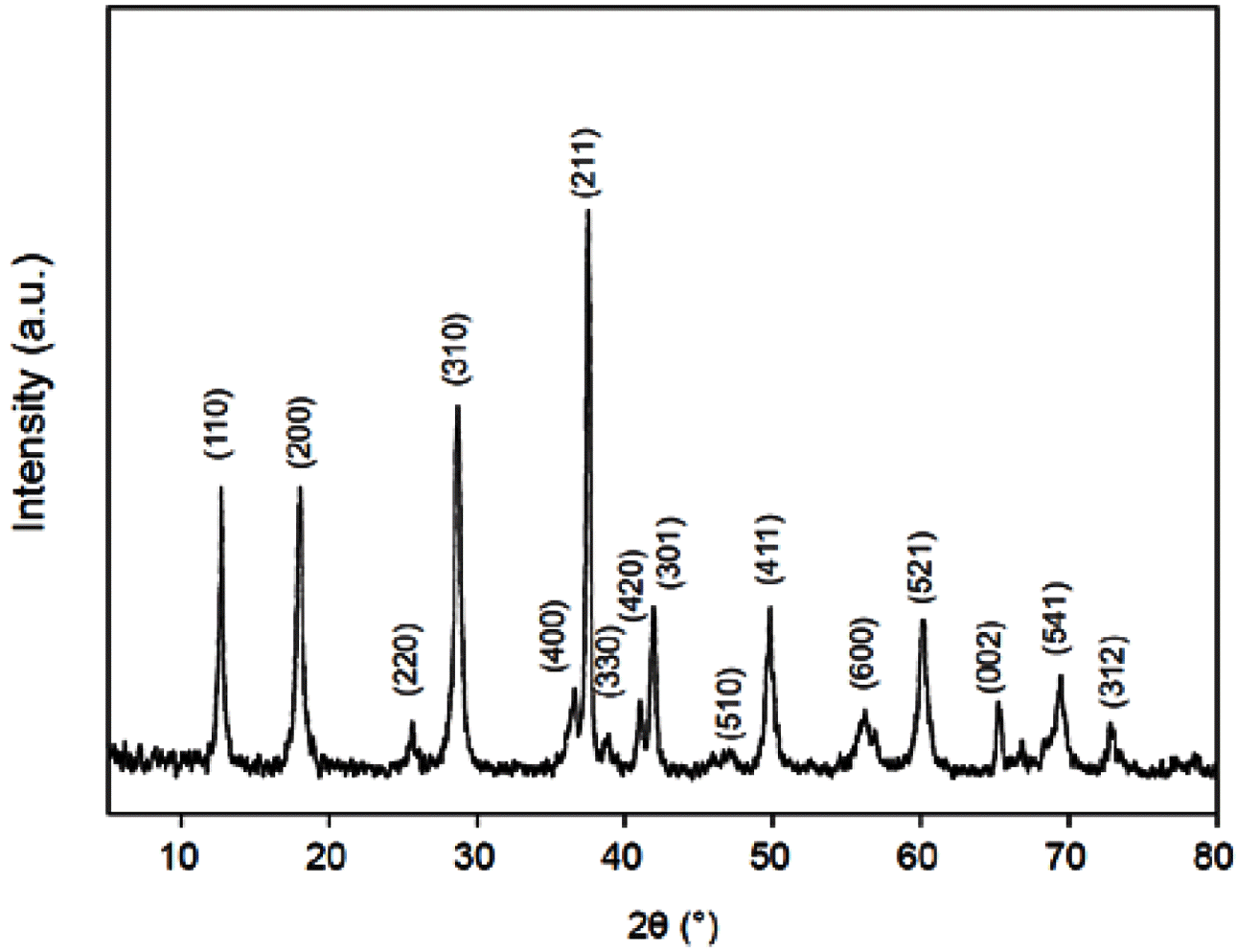
- The XRD pattern of MnO2/MC composites is shown in Figure 2. It is standard XRD spectra of α-type MnO2 (JCPDS 44-0141). No diffraction peaks of carbon are also observed in the XRD pattern, suggesting that α- MnO2 nanosheets was successfully grown on amorphous MCs. Nitrogen adsorption–desorption isotherms were carried out to estimate the specific surface area (SSA) and pore size distribution of the MnO2/MC composites. Figure 3(a) reveals typical type IV isotherms with hysteresis loops of type H3 (IUPAC classification) [14]. This hysteresis loop indicates that of the MnO2/MC compos- ites possess the slit-like mesopores [14]. The SSA of the MnO2/MC composites was calculated using multi-point BET method to be 376.16 m2/g. The MnO2/MC composites also have a narrow pore size distribution centered at 6.93 nm (Figure 3(b)). It is notable that the large surface area and hierarchical pores of the MnO2/MC composites would allow for enlarged accessible area and migration of molecules freely during catalytic reactions.
- (a) Nitrogen adsorption/desorption isotherms. (b) pore size distribution of the MnO2/MC composites.
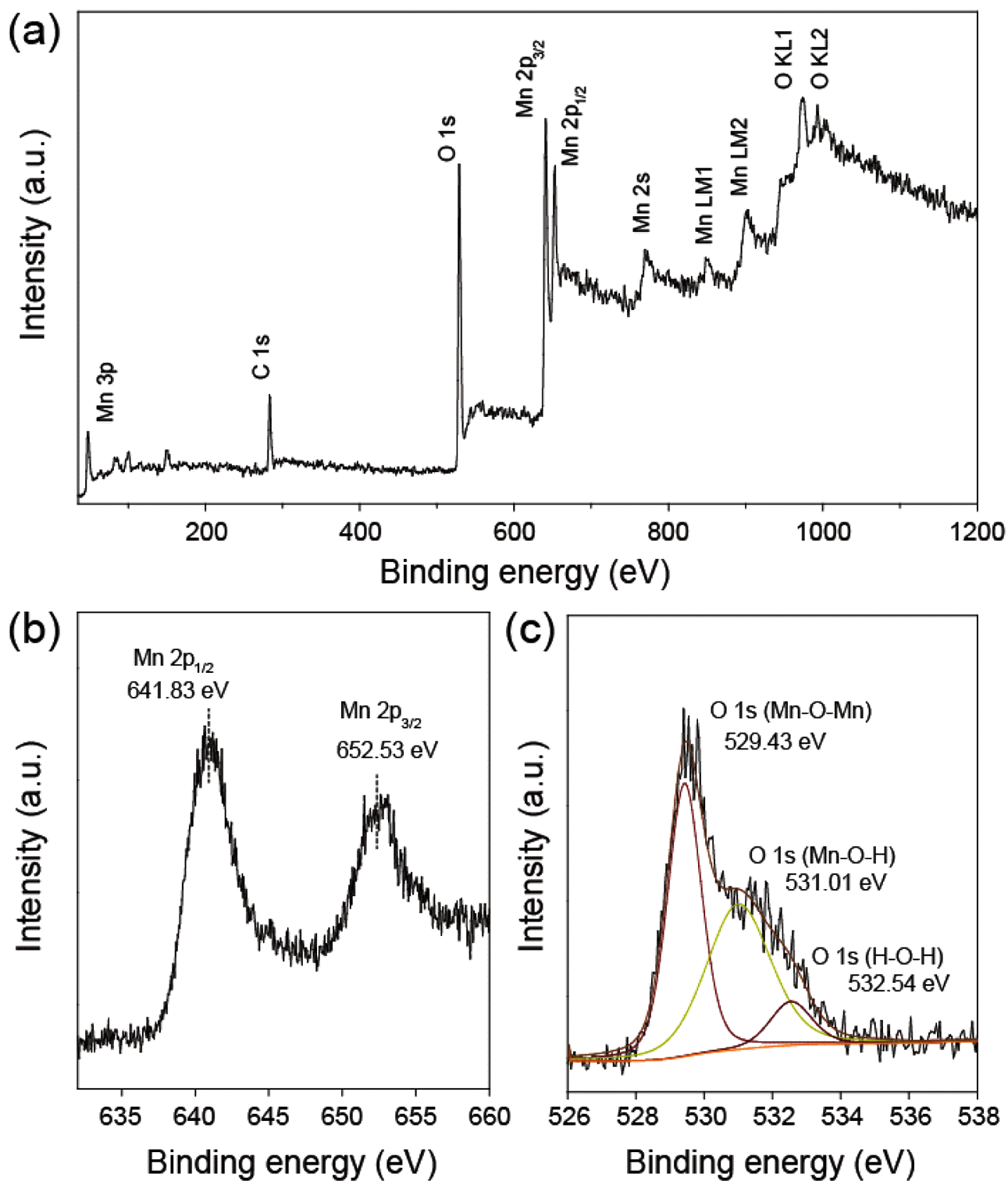
- Figure 4 represents XPS spectra of the MnO2/MC composites. Figure 4(a) presents the characteristic peak of C 1s, O 1s, and Mn 2p peaks, resulting from coexistence of carbon, oxygen, manganese elements. As shown in Figure 4(b), the high resolution spectrum of Mn 2p presents two major peaks centered at 641.83 eV and 652.53 eV with spin-orbital splitting of 11.7 eV, which is indicative of Mn 2p1/2 and Mn 2p3/2 of the manganese oxide [15]. The high resolution spectrum of O 1s has three distinct components (Figure 4(c)). A strong peak at 529.43 eV assigns to oxygen atoms bonded manganese in MnO2 [16]. The other peaks at 531.01 eV and 532.54 eV corresponds to oxygen atoms bonded hydroxyl group and absorbed water [16].
- (a) XPS survey spectrum and high resolution XPS spectra of (b) Mn 2p and (c) O 1s of the MnO2/MC composite
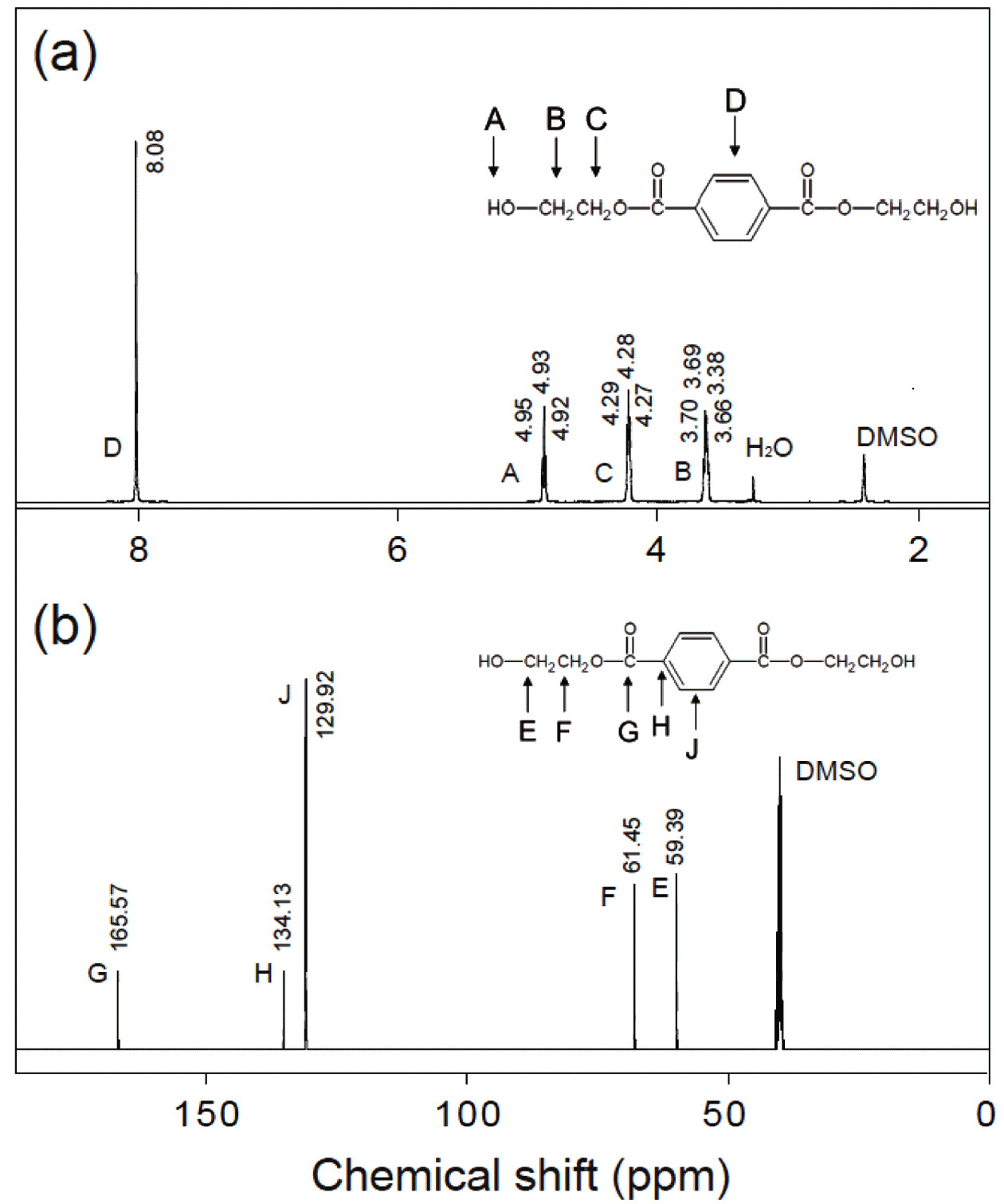
- The MCs-supported vertically-oriented MnO2 nanosheets can be used as a catalyst for the PET decomposition in eth- ylene glycol. The product after PET glycolysis catalyzed by the MnO2/MC composites was characterized using 1H and 13C NMR spectroscopy. Figure 5(a) shows the 1H NMR spectrum of the as-obtained product. The observed peak at δ 8.08 ppm is indicative of existence of H atoms related terephthalate. The peak located at δ 4.96 ppm corresponds to H atoms of hydroxyl end group. Two peaks located at δ 4.28 and δ 3.69 ppm correspond to the H atoms of methylene groups. This spectrum clearly provides peaks all present in BHET. The 13C NMR spectrum is also confirmed that the BHET was obtained after PET glycolysis with the MnO2/MC catalyst. Both NMR spectra are consistent with that reported in the literature [17,18].
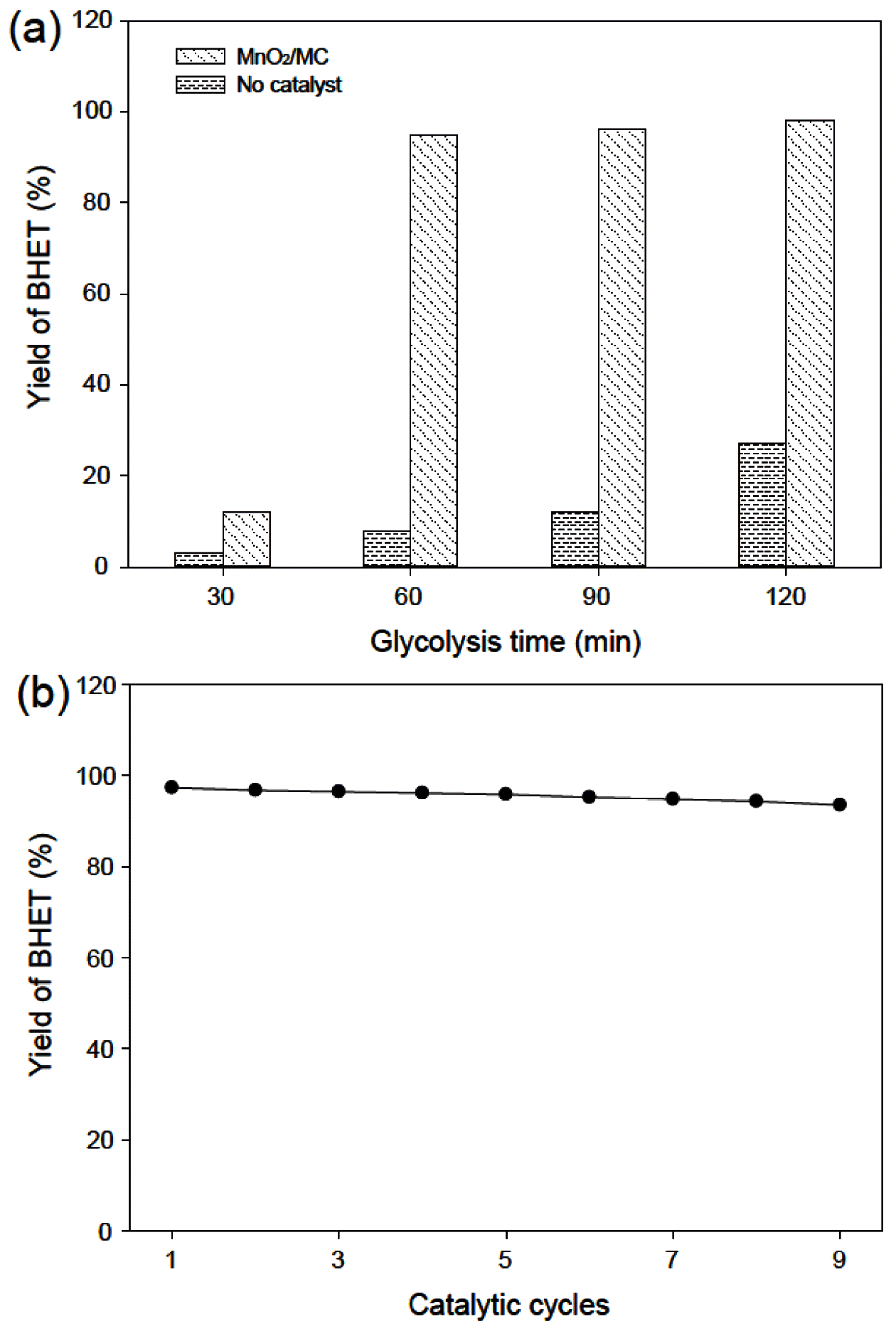
- Figure 6(a) shows the BHET yield as function of glycolysis time with and without a catalyst. In presence of the MnO2/MC catalyst, the BHET yield dramatically increased when increasing reaction time from 30 to 120 min and then finally reached 98% at 120 min. It is comparable results to other reported metal oxide-based catalysts, such as ZnMnO4 (92% at 60 min), (Mg-Zn)-Al LDH (75% at 180 min), GO-Mn3O4 (96.4% at 80 min) in glycolysis of PET [19,20,13]. However, the BHET yield is only 8% at reaction time of 30 min and then increased 27% at that of 120 min in the absence of catalysts. The MnO2/MC catalysts also have excellent reusability in PET glycolysis. As shown in Figure 6(b), the BHET yield slightly decreases even the ninth reuse of the MnO2/MC catalyst without regeneration process. Consequently, the MnO2/MC composites are efficient and reusable catalysts for PET glycolysis.
Results and Discussion
Fig. 3.
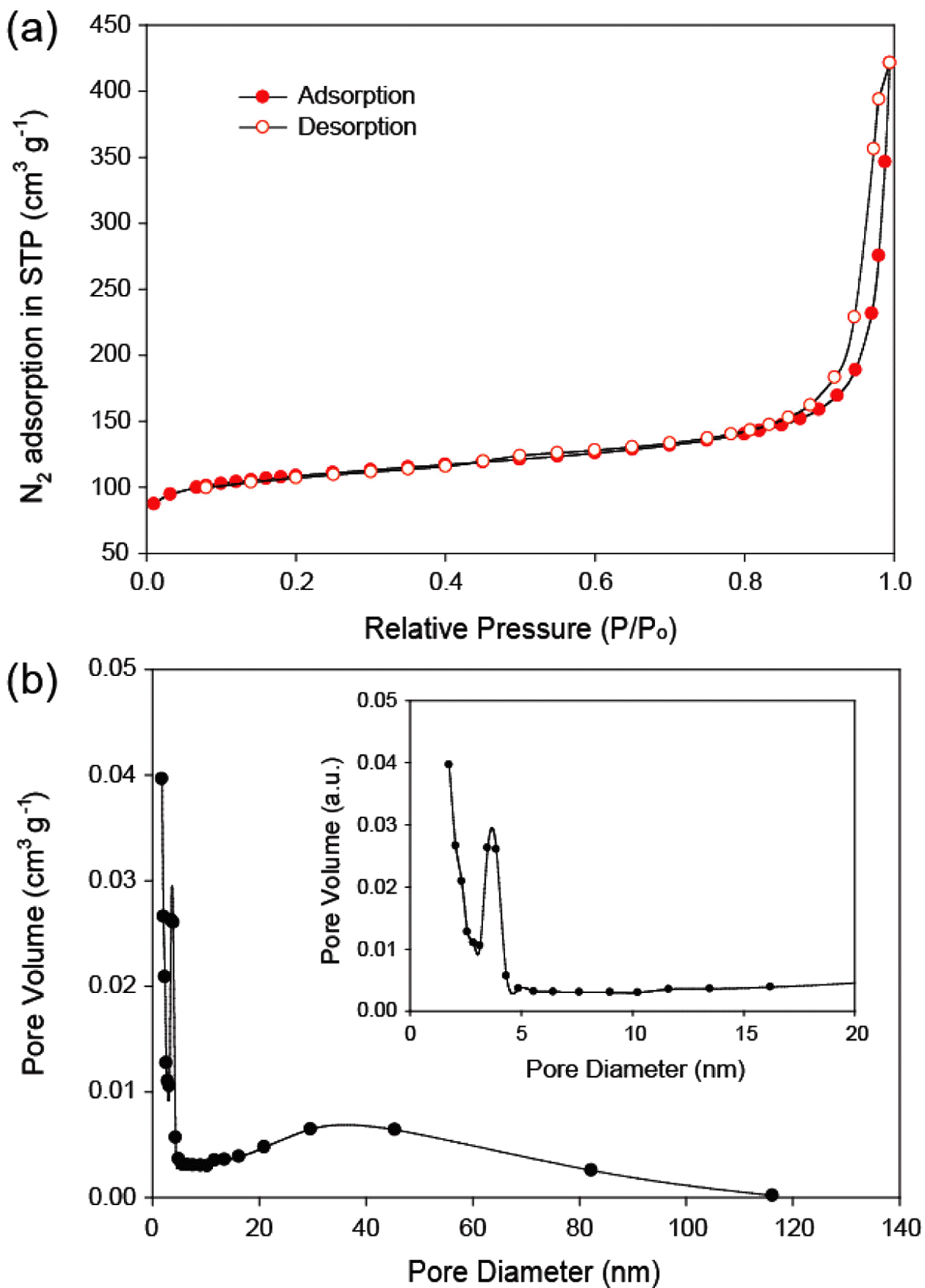

Fig. 4.

- Here, one-step hydrothermal approach to prepare the MnO2/MC composites as a catalyst for PET glycolysis. The macroporous MCs allow for full coverage of vertically- oriented MnO2 nanosheets with porous network, leading to a large surface area and easy access for molecules. In addition, the MnO2/MC composites represented the remarkable performance (i.e., high BHET yield (98% at 120 min and reusability (96.2% retention of the catalytic activity)) as a catalyst in PET decomposition. It is postulated that the hierarchical microstructure of the composites would lead to improved catalytic activity in PET glycolysis.
Conclusions
-
Acknowledgements
- This research was supported by The Leading Human Resource Training Program of Regional Neo industry through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2016H1D5A1910545).
Acknowledgment
- 1. R. Geyer, , J.R. Jambeck and and K.L. Law: : Sci. Adv.., 3 (2017) e1700782.
- 2. K. Müller, , E. Bugnicourt, , M. Latorre, , M. Jorda, , Y. E. Sanz, , J. M. Lagaron, , O. Miesbauer, , A. Bianchin, , S. Hankin, , U. Bölz, , G Pérez, , M. Jesdinszki, , M. Lindner, , Z. Scheuerer, , S. Castelló and and M. Schmid: : Nanomaterials., 7 (2017) 74.
- 3. C. M Rochman, , M. A Browne, , B. S. Halpern, , B. T. Hentschel, , E. Hoh, , H. K. Karapanagioti, , L. M. Rios-Mendoza, , H. Takada, , S. The and and R. C. Thompson: : Nature., 494 (2013) 169.
- 4. D. Xanthos and and T.R. Walker: : Mar. Pollut. Bull.., 118 (2017) 17.
- 5. B. Baytekin, , H.T. Baytekin and and B.A. Grzybowski: : Energy Environ. Sci.., 6 (2013) 3467.Article
- 6. A.M. Al-Sabagh, , F.Z. Yehia, , D.R.K. Harding, , G. Eshaq and and A.E. Elmetwally: : Green Chem.., 18 (2016) 3997.
- 7. J. Borda, , G. Pásztor and and M. Zsuga: : Polym. Degrad. Stabil.., 68 (2000) 419.
- 8. D. Kim, , B. Kim, , Y. Choi, , M. Han and and B-S. Kim: : Ind. Eng. Chem. Res.., 48 (2009) 685.Article
- 9. N. George and and T. Kurian: : Ind. Eng. Chem. Res.., 53 (2014) 14185.Article
- 10. F. Awaja and and D. Pavel: : Eur. Polym. J.., 41 (2005) 1453.
- 11. A.M. Al-Sabagh, , F.Z. Yehia, , G. Eshaq, , A.M. Rabie and and A.E. Elmetwally: : Egypt. J. Petrol.., 25 (2016) 53.
- 12. B. Hu, , K. Wang, , L. Wu, , S-H. Yu, , M. Antonietti and and M-M. Titirici: : Adv. Mater.., 22 (2010) 813.
- 13. G. Park, , L. Bartolome, , K.G. Lee, , S.J. Lee, , D.H. Kim and and T.J. Park: : Nanoscale., 4 (2012) 3879.Article
- 14. M. Thommes, , K. Kaneko, , A.V. Neimark, , J.P. Olivier, , F. Rodriguez-Reinoso, , J. Rouquerol and and K.S.W. Sing: : Pure Appl. Chem.., 87 (2015) 1051.
- 15. M. Liu, , W.W. Tjiu, , J. Pan, , C. Zhang, , W. Gao and and T. Liu: : Nanoscale., 6 (2014) 4233.Article
- 16. H-Q. Wang, , J. Chen, , S-J. Hu, , X-H. Zhang, , X-P. Fan, , J. Du, , Y-G. Huang and and Q-Y. Li: : RSC Advances., 5 (2015) 72495.
- 17. X. Song, , S. Zhang and and D. Zhang: : J. Appl. Polym. Sci.., 117 (2010) 3155.
- 18. Q. Wang, , X. Yao, , S. Tang, , X. Lu, , X. Zhang and and S. Zhang: : Green Chem.., 14 (2012) 2559.
- 19. M. Imran, , D.H. Kim, , W.A. Al-Masry, , A. Mahmood, , A. Hassan, , S. Haider and and S.M. Ramay: : Polym. Degrad. Stabil.., 98 (2013) 904.
- 20. G. Eshaq and and A.E. ElMetwally: : J. Mol. Liq.., 214 (2016) 1.
Figure & Data
References
Citations
Citations to this article as recorded by 

Preparation of the MnO2/Macroporous Carbon for PET Glycolysis
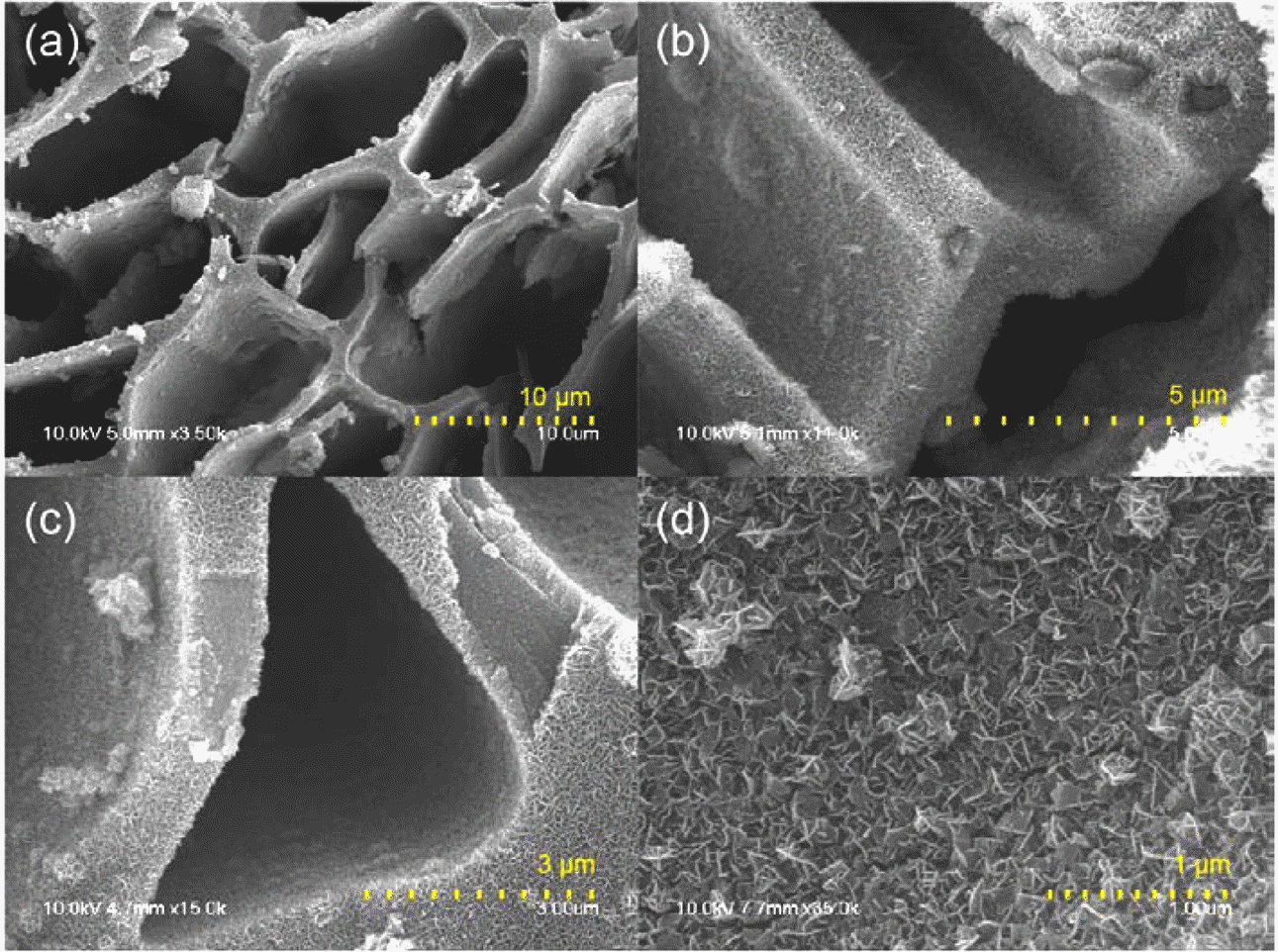
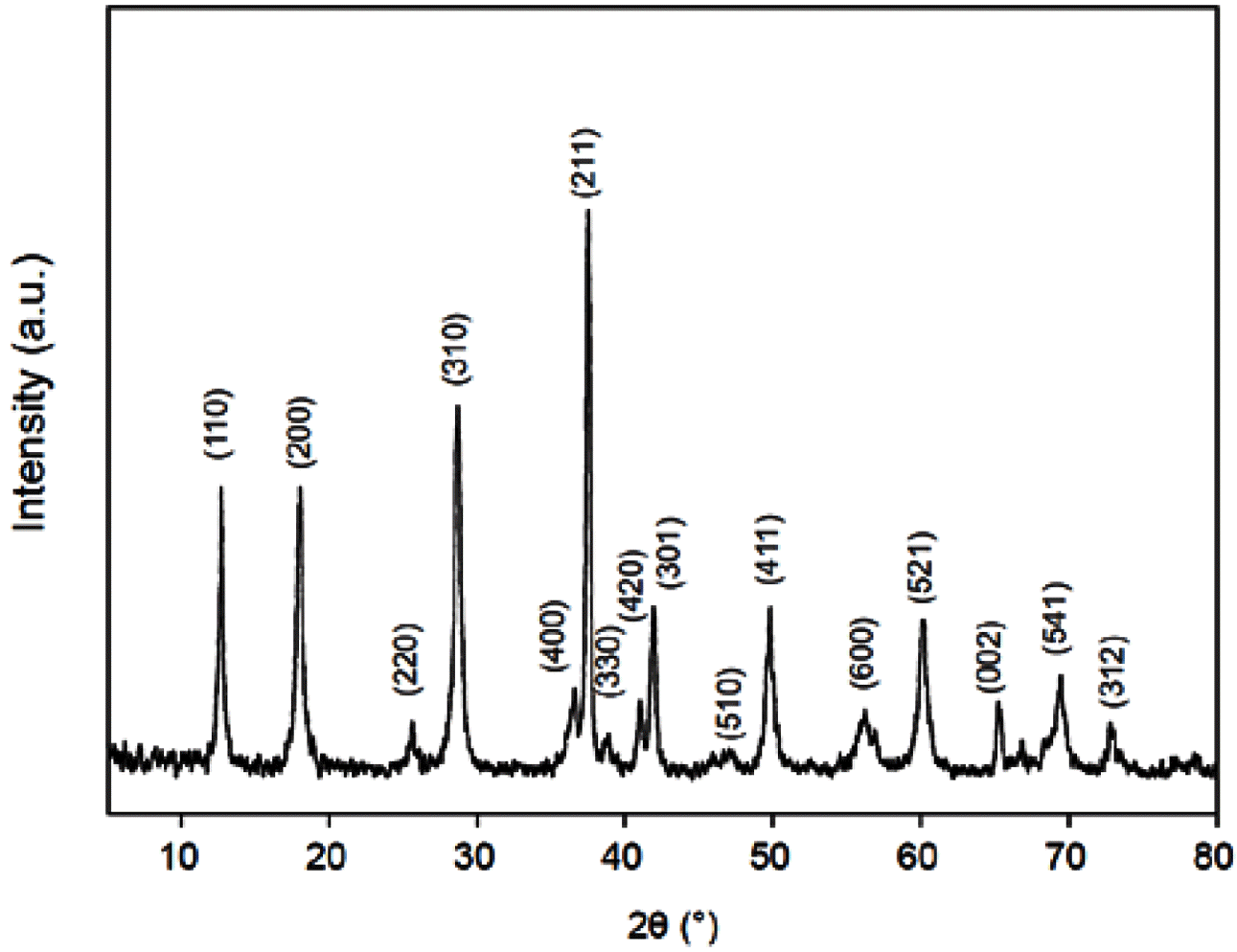
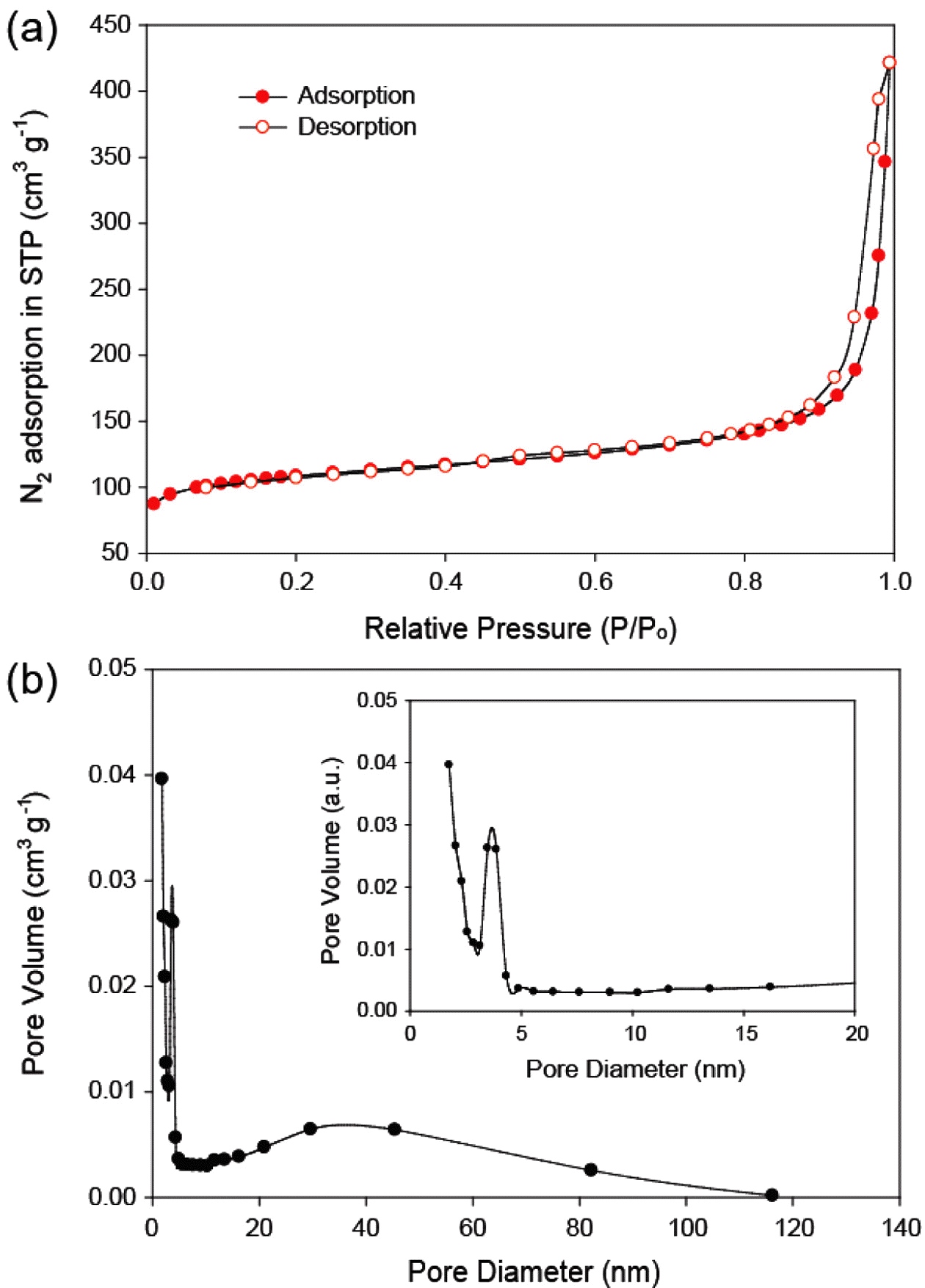
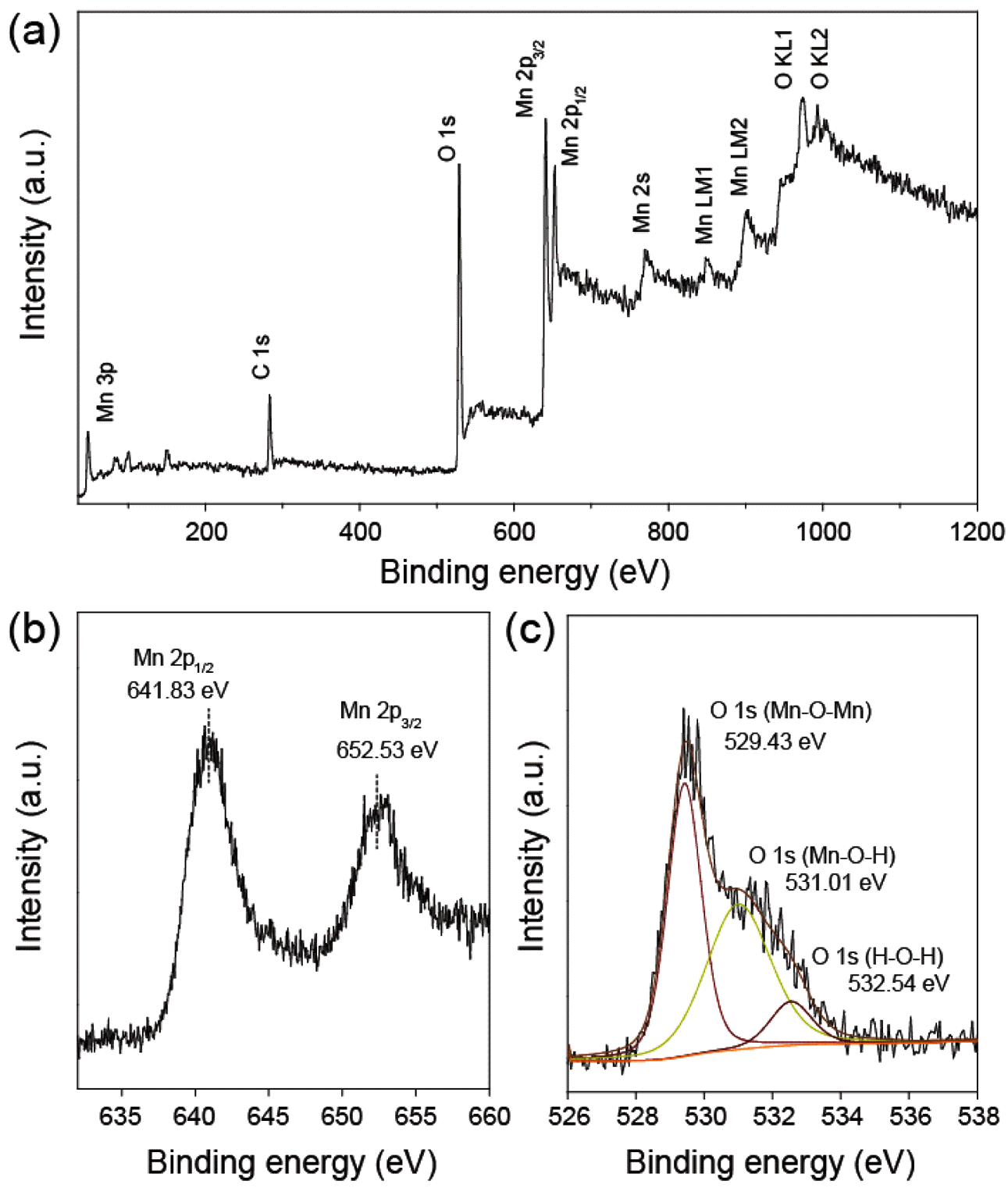
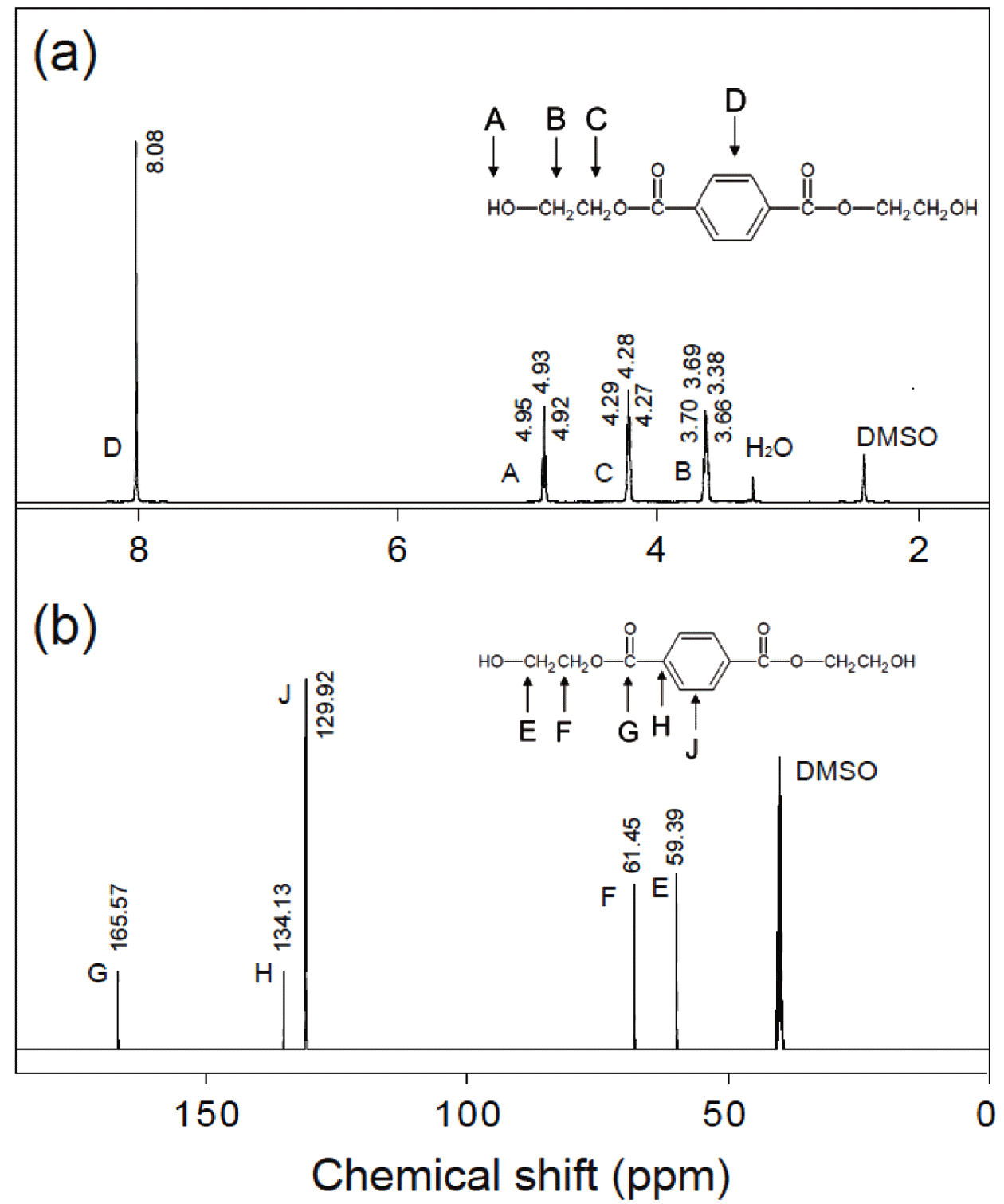
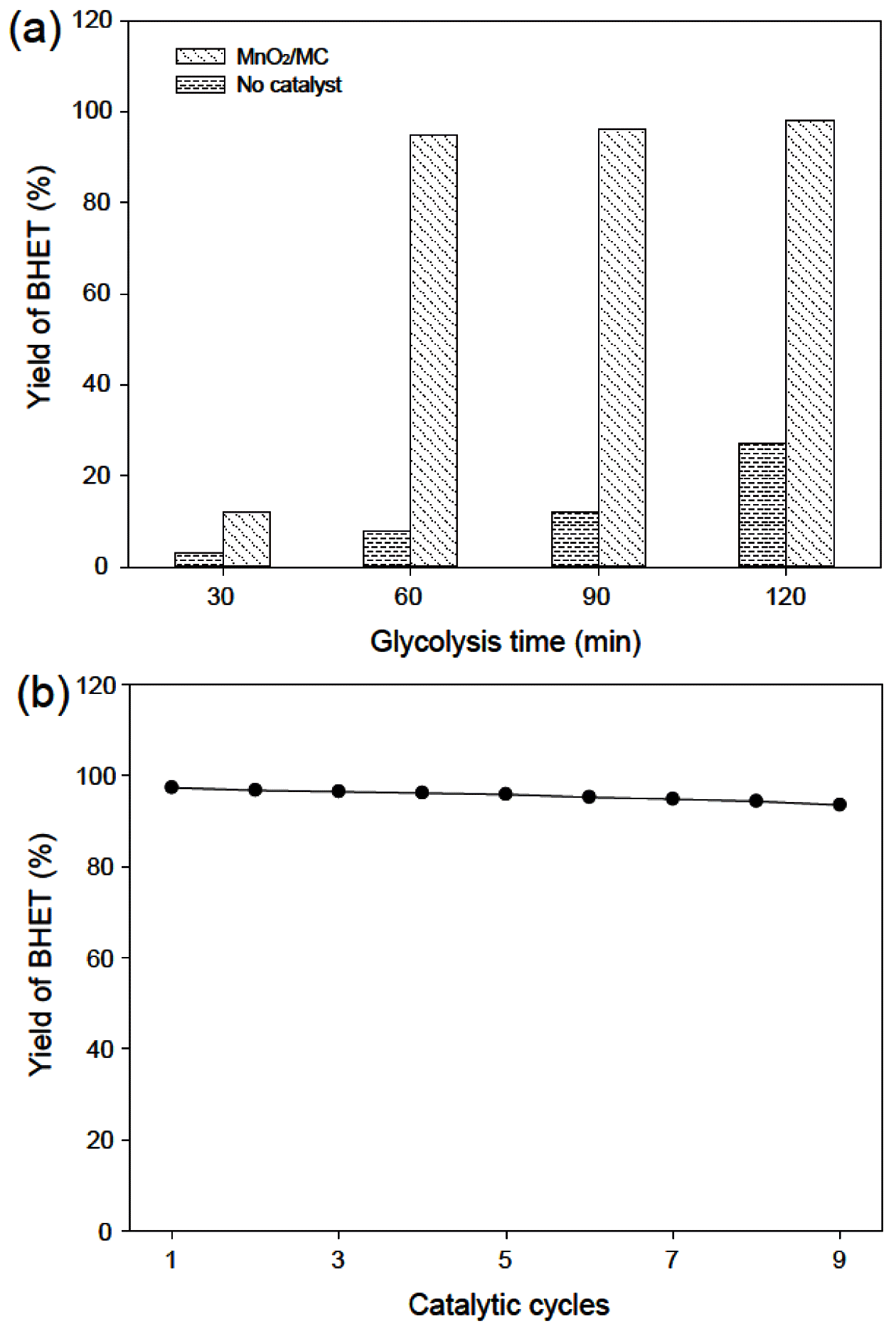
Fig. 1.
SEM images of the MnO2/MC composites with (a‒d) different magnification.
Fig. 2.
XRD pattern of the MnO2/MC composites.
Fig. 3.
(a) Nitrogen adsorption/desorption isotherms. (b) pore size distribution of the MnO2/MC composites.
Fig. 4.
(a) XPS survey spectrum and high resolution XPS spectra of (b) Mn 2p and (c) O 1s of the MnO2/MC composite
Fig. 5.
(a) 1H NMR and (b) 13C NMR spectrum of as-produced BHET.
Fig. 6.
(a) BHET yield with increasing glycolysis time. (b) Recycling of the MnO2/MC catalysts.
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
Preparation of the MnO2/Macroporous Carbon for PET Glycolysis
TOP
 KPMI
KPMI

 Cite this Article
Cite this Article






