Articles
- Page Path
- HOME > J Korean Powder Metall Inst > Volume 25(4); 2018 > Article
-
ARTICLE
- Development of Carbon Nanotube-copper Hybrid Powder as Conductive Additive
- Minjae Leea, Seoungjun Haaa, Yeonjoo Leeb, Haneul Jangb, Hyunjoo Choib,*
-
Journal of Korean Powder Metallurgy Institute 2018;25(4):291-295.
DOI: https://doi.org/10.4150/KPMI.2018.25.4.291
Published online: July 31, 2018
a Seoul Science High School, Seoul, KS013, Republic of Korea
b Dept. of Advanced Materials Engineering, Kookmin University, Seoul, KS013, Republic of Korea
- *Corresponding Author: TEL: +82-2-910-4287, FAX: +82-2-910-4320, E-mail: hyunjoo@kookmin.ac.kr
• Received: August 17, 2018 • Revised: August 20, 2018 • Accepted: August 21, 2018
© The Korean Powder Metallurgy Institute. All rights reserved.
- 1,347 Views
- 1 Download
- 1 Crossref
Abstract
- A conductive additive is prepared by dispersing multi-walled carbon nanotubes (MWCNTs) on Cu powder by mechanical milling and is distributed in epoxy to enhance its electrical conductivity. During milling, the MWCNTs are dispersed and partially embedded on the surface of the Cu powder to provide electrically conductive pathways within the epoxy-based composite. The degree of dispersion of the MWCNTs is controlled by varying the milling medium and the milling time. The MWCNTs are found to be more homogeneously dispersed when solvents (particularly, non-polar solvent, i.e., NMP) are used. MWCNTs gradually disperse on the surface of Cu powder because of the plastic deformation of the ductile Cu powder. However, long-time milling is found to destroy the molecular structure of MWCNTs, instead of effectively dispersing the MWCNTs more uniformly. Thus, the epoxy composite film fabricated in this study exhibits a higher electrical conductivity than 1.1 S/cm.
- Carbon nanomaterials are being explored as replacements for expensive conducting additives such as gold or silver nanoparticles [1, 2]. Carbon nanomaterials, such as carbon nanotubes (CNTs) or graphene, are known to significantly enhance the electrical conductivity of polymerbased composites [3-5], because of their exceptional intrinsic electrical properties like high current density [6] and high aspect ratio [7]. However, they easily tend to agglomerate because of the Van der Waals force that acts on their large surface area [8].
- The morphology of conductive hybrids has been reported to play a significant role in determining the conductivities of polymer-based composites [9]. Hence, it is necessary to uniformly disperse carbon nanomaterials in polymer-based composites. Solution-based processes, which involve ultrasonication with the help of a surfactant, have been widely utilized to achieve uniform dispersion of carbon nanomaterials [10, 11]. The best solvent to disperse CNTs is amide, especially N,N-dimethylformamide and N-methyl-2-pyrrolidone (NMP) [12]; solvents that do not contain hydrogen donors and exhibit Lewis basicity are known to be highly effective in dispersing CNTs [13]. However, these solution-based methods may significantly damage the molecular structure of CNTs and have limitations in mass production [14].
- In this study, copper/multi-walled CNT (MWCNT) hybrids are proposed as conductive additives for polymer- based composites. Compared to carbon nanomaterials, micro-sized hybrids can be more easily dispersed in polymer-based composites. Furthermore, MWCNTs being physically attached to the surface of Cu powder can provide pathways for electrons by acting as bridges between the Cu powder to reduce the distance from one particle to another without increasing the amount of heavy copper used. Mechanical milling was employed as a facile process to produce large amounts of hybrids in a batch. The milling condition was controlled by varying the milling time and the milling medium. Dry milling and wet mill- ing using NMP and toluene as polar and non-polar solvents were conducted to investigate the effect of degree of dispersion of MWCNTs on the electrical conductivity of the final composites containing the hybrids.
Introduction
- A variety of Cu/MWCNTs hybrids were produced using a planetary mill (Pulverisette 5 classic line, Fritsch ™, Germany) at a fixed milling speed of 100 rpm, but by varying the milling time and the solvent. Prior to milling, 20.9 g of Cu (≥ 99.9% purity, 30 μm in mean diameter) and 9.1 g of MWCNTs (≥ 90%) were placed in 500 mL stainless chamber to obtain Cu/CNT hybrid at a volume ratio of 1:3; the volume ratio was calculated with an assumption that densities of Cu and MWCNTs are 8.9 and 1.3 g/cc, respectively. Four hundred fifty grams of stainless steel balls (5 mm in diameter) were used as the milling medium, and the ball/powder weight ratio was 15:1. To vary the milling time, 5 samples were prepared at different milling time (0.5, 1, 3, 6, and 9 h) under dry conditions in argon atmosphere. For comparison, wet milling was conducted for 1 h with NMP (≥ 99.5%, Samchun, Korea) as the polar solvent and toluene (≥ 99.5%, Samchun, Korea) as the non-polar solvent.
- Epoxy-based composites were prepared using the hybrids; 1 g of epoxy (bisphenol A, YD-128, Kukdo Chemical Co., Ltd, Korea), 1 g of epoxy hardener (Cyclohexanemethanamine, KH-700, Kukdo Chemical Co., Ltd, Korea), and 1 g of distilled water were taken in a beaker and pre-heated to 80°C. The hybrids were added into the beaker at specific volume ratios (30, 40, and 50 vol%). The mixture was flattened to approximately 5- 6 mm thickness and dried in an oven (Over ON-01E, JEIO TECH) at 80°C for 3 h.
- The microstructures of the hybrids and the epoxy-based composites were analyzed using a scanning electron microscope (SEM, JEM-7610F, JEOL Ltd., Japan) and an optical microscope (OM, SMZ 745, Shivani Scientific Industrial Ltd, India). The molecular structure of the MWCNTs was identified by Raman spectroscopy (Raman, LabRam HR, HORIBA Jobin Yvon Co. Ltd., France). The electrical conductivity of the epoxy-based composites was calculated by measuring the electrical resistivity of the composites using a 4-point probe (Keithley 2400, Tektronix, Korea).
Experimental
- Figure 1 shows the SEM images of the Cu/MWCNT hybrids produced by ball milling without solvents at milling time of 0.5, 1, 3, 6, and 9 h. With increase in the milling time, the Cu powder became flattened, and thread-like MWCNTs were dispersed and partially embedded in the Cu powder. Figure 1 (f) shows the ratio of the surface area of Cu covered with MWCNTs to the free Cu surface area, estimated from the SEM images. The increase in the area covered by MWCNTs with increase in the milling time is attributed to the increase in the degree of dispersion of MWCNTs; the aggregation of MWCNTs became less severe as the milling time was increased. Before milling, some of the MWCNT bundles were held together by the Van der Waals force acting on the large surface area of the MWCNTs. During mechanical milling, ductile Cu powder is plastically deformed, becoming flattened, and MWCNTs can be dispersed via the plastic deformation of the Cu powder. After 1 h of milling, most of MWCNTs appear to be dispersed on the surface of the Cu powder. Longer ball milling was found to destroy the molecular structure of the MWCNTs rather than effectively dispersing the MWCNTs more uniformly.
- We conducted ball milling for a fixed milling time of 1 h, which was considered to be the most appropriate for distributing CNTs [15], using different solvents; milling was conducted under i) dry condition in argon atmosphere and ii) wet condition with NMP as the polar solvent or toluene as the non-polar solvent. Figure 2 shows the SEM images of the Cu/MWCNT hybrids produced by ball milling for a fixed milling time of 1 h using different solvents and the ratio of the surface area of Cu covered with CNTs to the free Cu surface area, estimated from the SEM images. The MWCNTs were found to be more homogeneously dispersed when solvents (particularly, polar solvent, i.e., NMP) were used; however, MWCNTS were well dispersed in the Cu/MWCNT hybrids prepared at all the conditions.
- The Raman spectra of the Cu/MWCNT hybrids produced by ball milling for a fixed milling time of 1 h under dry condition and wet condition with NMP and toluene show three distinct peaks as shown in Fig. 3. The D-band, observed at approximately 1350 cm-1, originates from lattice defects and corresponds to the breathing modes of sp2 atoms in rings, and the G-band, observed at approximately 1575 cm-1, originates from perfect hexagonal graphite and corresponds to the bond-stretching of all pairs of sp2 atoms in both rings and chains [16]. Hence, the G band is an indicator of in-plane stretching of carbon bonds, while the D band indicates the defect state of MWCNT surfaces. The defects induced during milling result in peak shifts of the G-band towards larger wave numbers [17]. Therefore, the ratio between these two bands (D/G ratio) indicates the overall quality of MWCNTs. The D/G ratio does not vary significantly in all the samples, suggesting that mechanical ball milling may damage the quality of MWCNT to some extent, but the effect is similar for the three different conditions. Thus, the quality of the Cu-MWCNT hybrids in terms of quality of MWCNTs does not vary appreciably.
- The hybrids were well dispersed in the epoxy-based composites, as shown in Fig. 4. The Cu particles are visible (white color) when milling is conducted with NMP.
- The high degree of dispersion indicates that when CNTs are physically attached to Cu powder, the drawback of CNT self-aggregation and localization has been overcome. Most of the hybrids were randomly dispersed in the epoxy matrix possibly without making physical contact with each other; but, the well-dispersed MWCNTs on the surface of the Cu powder could form a conductive network by close connection.
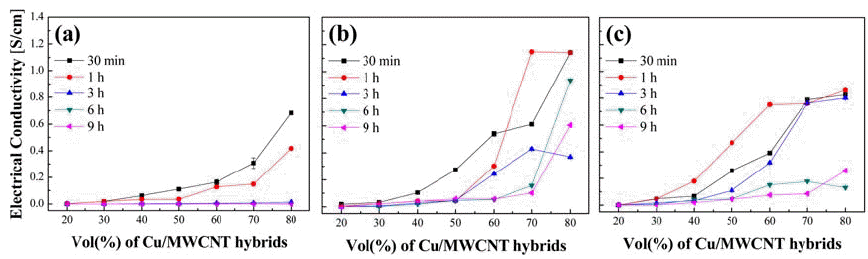
- Figure 5 reveals the electrical conductivities of the epoxy-based composites containing Cu/MWCNT hybrids produced by ball milling using different solvents. It is observable that the electrical conductivity increases in accordance with the volume percentage of the hybrid Cu- CNT powder. This phenomenon is attributed to improved connection between the Cu particles. According to the percolation theory, the electrical conductivity of a conductible material will increase significantly when conductible particles begin to have direct contact with each other [18]. It seems that a volume percentage of 40 to 60% powder is the critical concentration for significant conductivity enhancement. Beyond this concentration, there is no significant enhancement in the electric conductivity with the volume percentage of the powder. Fur thermore, the physical property of the resulting epoxy filler resembles crumb lumps rather than fillers. Thus, it is not practical to prepare highly powder-dense mixtures. It is also shown that the electrical conductivity is significantly enhanced when solvents are used in the milling process (Fig. 5 (b) and (c)). Furthermore, composites with 50 vol% fillers milled with NMP (Fig. 5 (b)) as the solvent showed approximately 5 times better electrical conductivity than those milled with toluene (Fig. 5 (c)). It is known that even traces of residue solvent used during nanotube bundle-reinforced epoxy nanocomposite preparation has notable effects on the physical properties of the total composite [19]. In addition, solvents that disperse CNT well are suggested to have Hansen solubility parameters in a specific range. Both NMP and toluene have a Hansen dispersion component of 18.0 MPa1/2 which fits the range proposed by former studies. However, NMP is a solvent well known for CNT dispersion, whereas toluene only contributes to a swollen dispersion state of CNT when CNT is mixed and left for long periods in toluene [20]. We deduce that solvents that disperse CNT well will improve the attachment of CNT to Cu, thus resulting in better connection of Cu-CNT particles dispersed throughout the epoxy mixture. Furthermore, we predict that solvents will guide CNT attached to different Cu particles to approach each other as the solvent dries during the drying process. Therefore powders prepared with appropriate solvents will make a better connection between Cu particles when dispersed in an epoxy mixture.
Results and Discussion
Fig. 1
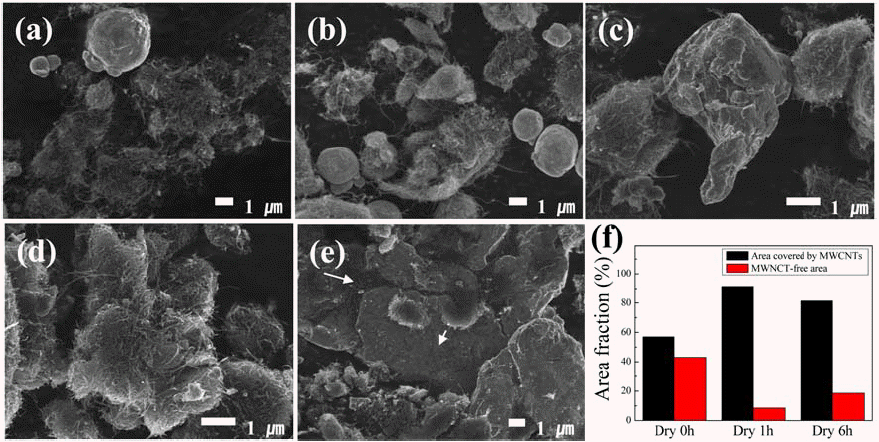
SEM images of Cu/MWCNT hybrids produced by ball milling without solvents at a milling time of (a) 30 minutes, (b) 1, (c) 3, (d) 6 and (e) 9 hours. (f) reveals the area ratio of Cu surface area covered with CNTs to free Cu surface area, estimated on the basis of SEM images.

Fig. 2
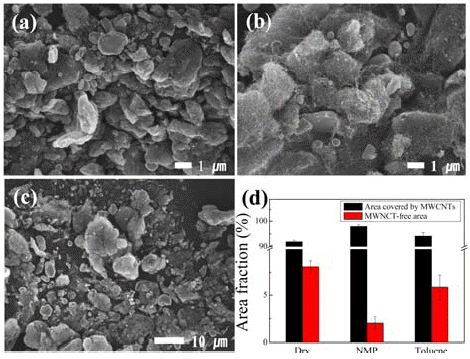
SEM images of Cu/MWCNT hybrids produced by ball milling for a fixed milling time of 1 hour (a) under dry condition and wet condition (b) with NMP and (c) Toluene. (d) indicates the area ratio of Cu surface area covered with CNTs to free Cu surface area, estimated on the basis of SEM images.

Fig. 3
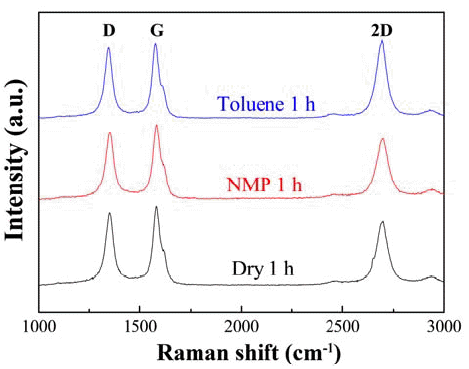
Raman spectra of Cu/MWCNT hybrids produced by ball milling for a fixed milling time of 1 hour under dry condition and wet condition with NMP and Toluene.

Fig. 4
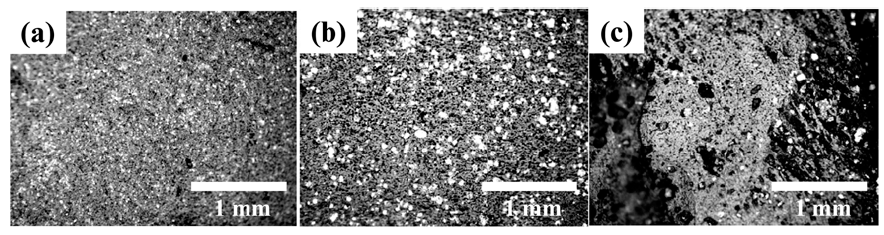
OM images of the polished surface of epoxy-based composites containing Cu/MWCNT hybrids produced by ball milling for a fixed milling time of 1 hour under (a) dry condition and wet condition with (b) NMP and (c) Toluene.

- A conductive additive was developed by dispersing MWCNTs on Cu powder by mechanical milling, which was distributed in epoxy to enhance its electrical conductivity. The degree of dispersion of MWCNTs was controlled by varying the milling medium and the milling time. In the initial stage of milling, MWCNTs were gradually dispersed on the surface of Cu powder because of plastic deformation of ductile Cu powder. However, longtime milling was found to destroy the molecular structure of MWCNTs rather than effectively dispersing MWCNTs more uniformly. Solvents NMP and toluene were both capable of increasing the degree of dispersion of CNT on Cu surfaces. Raman spectra indicated that there was no significant difference in the quality degradation of CNT through mechanical milling under dry and wet conditions (regardless of the type of solvent). The OM images demonstrated that the hybrids prepared with different solvents were dispersed well in the epoxy-based composites. When toluene was used as the solvent, the resulting film showed excellent electrical conductivity; however, with NMP as the solvent, the electrical conductivity of the composite was even better. The MWCNTs were dispersed and partially embedded on the surface of Cu powder during milling to provide electrically conducting pathways inside the epoxy-based composite.
Conclusions
-
Acknowledgements
- This paper was supported by the grant of the Seoul Science High School. This work was partially supported by through the National Research Foundation (NRF) of Korea (2015R1A5A7037615). This research was also supported by Civil-Military Technology Cooperation Program (18-CM-MA-15).
Acknowledgements
- [1] . P. C. Ma , B. Z. Tang and J. K. Kim: Carbon., 46 (2008) 14970008-6223.Article
- [2] . D. Zhao , T. Liu, J. G. Park, M. Zhang, J. M. Chen and B. Wang: Microelectron. Eng.., 96 (2012) 710167-9317.Article
- [3] . P. M. Ajayan , O. Stephan, C. Colliex and D. Trauth: Science., 265 (1994) 12120036-8075.ArticlePubMed
- [4] . O. Lourie , D. M. Cox and H. D. Wagner: Phys. Rev. Lett.., 81 (1998) 16380031-9007.Article
- [5] . L. S. Schadler , S. C. Giannaris and P. M. Ajayan: Appl. Phys. Lett.., 73 (1998) 38420003-6951.Article
- [6] . S. G. Shin : Met. Mater. Int.., 7 (2001) 6051598-9623.Article
- [7] . Y. Ando , X. Zhao, H. Shimoyama, G. Sakai and K. Kaneto: Int. J. Inorg. Mater.., 1 (1999) 771466-6049.Article
- [8] . T. Uchida and S. Kumar: J. Appl. Polym. Sci.., 98 (2005) 9850021-8995.Article
- [9] . S. Berber , Y. K. Kwon and D. Tomanek: Phys. Rev. Lett.., 84 (2000) 46130031-9007.ArticlePubMed
- [10] . Y. Y. Huang and E. M. Terentjev: Polymer., 4 (2012) 2750032-3861.
- [11] . Y. Y. Huang and E. M. Terentjev: Adv. Funct. Mater.., 20 (2010) 40621616-301X.Article
- [12] . Hoboken: Solution processing of inorganic materials, In : . Wiley, Canada (2009) 511.
- [13] . K. D. Ausman , R. Piner, O. Lourie, R. S. Ruoff and M. Korobov: J. Phys. Chem. B., 104 (2000) 89111520-6106.Article
- [14] . J. Liu , A. G. Rinzler, H. Dai, J. H. Hafner, R. K. Bradley, P. J. Boul, A. Lu, T. Iverson, K. Shelimov, C. B. Huffman, F. R. Macias, Y. S. Shon, T. R. Lee, D. T. Colbert and R. E. Smalley: Science., 280 (1998) 12530036-8075.ArticlePubMed
- [15] . H. J. Choi , J.H. Shin and D.H. Bae, (2012.
- [16] . C. Casiraghi , A. C. Ferrari and J. Robertson: Phys. Rev. B., 72 (2005) 854011098-0121.Article
- [17] . S. Berber and A. Oshiyama: Physica B., 376 (2006) 2720921-4526.Article
- [18] . N. Hu , Y. Karube, C. Yan, Z. Masuda and H. Fukunag: Acta Mater.., 56 (2008) 29291359-6454.Article
- [19] . K. T. Lau , M. Lu, C. K. Lam, H. Y. Cheung, F. L. Sheng and H. L. Li: Compos. Sci. Technol.., 65 (2005) 7190266-3538.Article
- [20] . H. T. Ham , Y. S. Choi and I. J. Chung: J. Colloid Interf. Sci.., 286 (2005) 2160021-9797.ArticlePubMed
Figure & Data
References
Citations
Citations to this article as recorded by 

- Effects of Morphologies of Carbon Nanomaterials on Conductivity of Composites Containing Copper/Carbon Nanomaterial Hybrid Fillers
Yeonjoo Lee, Sung-uk Hong, Hyunjoo Choi
Journal of Korean Powder Metallurgy Institute.2018; 25(5): 435. CrossRef
Development of Carbon Nanotube-copper Hybrid Powder as Conductive Additive

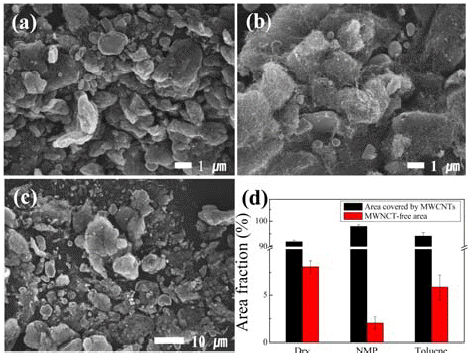



Fig. 1
SEM images of Cu/MWCNT hybrids produced by ball milling without solvents at a milling time of (a) 30 minutes, (b) 1, (c) 3, (d) 6 and (e) 9 hours. (f) reveals the area ratio of Cu surface area covered with CNTs to free Cu surface area, estimated on the basis of SEM images.
Fig. 2
SEM images of Cu/MWCNT hybrids produced by ball milling for a fixed milling time of 1 hour (a) under dry condition and wet condition (b) with NMP and (c) Toluene. (d) indicates the area ratio of Cu surface area covered with CNTs to free Cu surface area, estimated on the basis of SEM images.
Fig. 3
Raman spectra of Cu/MWCNT hybrids produced by ball milling for a fixed milling time of 1 hour under dry condition and wet condition with NMP and Toluene.
Fig. 4
OM images of the polished surface of epoxy-based composites containing Cu/MWCNT hybrids produced by ball milling for a fixed milling time of 1 hour under (a) dry condition and wet condition with (b) NMP and (c) Toluene.
Fig. 5
Electrical conductivities of epoxy-based composites containing Cu/MWCNT hybrids produced by ball milling for a fixed milling time of 1 hour under (a) dry condition and wet condition with (b) NMP and (c) Toluene.
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
Development of Carbon Nanotube-copper Hybrid Powder as Conductive Additive
TOP
 KPMI
KPMI

 Cite this Article
Cite this Article





