Articles
- Page Path
- HOME > J Powder Mater > Volume 31(5); 2024 > Article
-
Research Article
- Recovery of Barium, Nickel, and Titanium Powders from Waste MLCC
- Haein Shin, Kun-Jae Lee*
-
Journal of Powder Materials 2024;31(5):374-381.
DOI: https://doi.org/10.4150/jpm.2024.00192
Published online: October 31, 2024
Department of Energy Engineering, Dankook University, Cheonan 31116, Republic of Korea
- *Corresponding Author: Kun-Jae Lee TEL: +82-41-550-3684 FAX: +82-41-559-7945 E-mail: kjlee@dankook.ac.kr
© The Korean Powder Metallurgy & Materials Institute
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,561 Views
- 43 Download
Abstract
- The development of the electronics industry has led to an increased demand for the manufacture of MLCC (Multilayer Ceramic Capacitors), which in turn is expected to result in a rise in MLCC waste. The MLCC contains various metals, notably barium, titanium, and nickel, whose disposal is anticipated to increase correspondingly. Recently, recycling technologies for electronic waste have garnered attention as they address waste management and raw material supply challenges. This paper investigates the recovery of barium, nickel, and titanium from the MLCC by a hydrometallurgical process. Using citric acid, which is an organic acid, the metal inside the MLCC was leached. Additionally, metal materials were recovered through precipitation and complexing processes. As a result, barium and titanium were recovered from the leachate of the waste MLCC, and 93% of the nickel-based powder was recovered. Furthermore, the optimal recovery process conditions for recycling these metal elements were investigated.
- The mineral resources we currently rely on are entirely imported. As these resources are depleting, recycling materials from electronic waste and industrial waste is anticipated to become a primary source [1]. In contrast to the finite nature of these resources, the electronics industry is rapidly advancing. The exponential growth in electronic waste, driven by increasing demand for electronic products, is expected to continue. Thus, recycling the rapidly growing electronic waste is imperative to solve environmental issues and ensure a stable supply of resources, necessitating the development of recycling processes. One of the essential components in the electronics industry, the Multilayer Ceramic Capacitor (MLCC), is increasingly replacing traditional capacitors due to its compact size. As the demand for miniaturization and lightweight electronic devices grows, MLCC provides a significant advantage by reducing the volume of electronic products. Consequently, they are extensively applied across various electronic applications [2]. MLCC is widely used in nearly all products containing semiconductors and electronic circuits, with a minimum of 1,000 units used in smartphones and displays and over 10,000 units in electric vehicles [3]. Therefore, it is necessary to supply raw materials by recycling waste MLCC and simultaneously address disposal issues. However, while recycling technologies for recovering valuable metals from electronic components such as PCBs and batteries have been researched, the technology for recycling MLCC remains underdeveloped.
- MLCC contains approximately 50% barium titanate (BaTiO3) as the dielectric material and around 15% nickel (Ni) as the internal electrode [4, 5]. The Barium (Ba), Titanium (Ti), and Ni elements present in MLCCs are considered high-value resources. Barium compounds are utilized in various applications, such as automotive road materials and contrast agents [6]. Titanium is known for its high strength-to-density ratio, thermal resistance, and corrosion resistance. It is extensively employed in the aerospace industry and chemical and optical devices [7]. Nickel is a high-value material applied in numerous optical and electronic industries, including display charge transport layers, battery materials, and gas sensors [8]. The demand for these metallic materials has significantly increased due to the rapid industrial development. However, due to the high dependency on imports, the supply of these materials remains insufficient. Therefore, research on recycling technologies to ensure material self-sufficiency and a stable supply of Ba, Ni, and Ti is essential [9].
- The hydrometallurgical processes involve crushing, leaching, solvent extraction, and precipitation to recover metals. Generally, inorganic acids such as sulfuric acid, nitric acid, and hydrochloric acid are used as leaching agents for metal leaching [10]. Using these inorganic acids emits toxic gases such as Cl2, SO3, and NOx, and necessitates secondary wastewater treatment [11]. The additional processing costs to mitigate wastewater and air pollution make these methods economically and environmentally unsustainable. Research into metal leaching using organic acids is necessary to address the disadvantages of inorganic acid leaching. Organic acids are easy to manage due to their biodegradable properties and not emitting harmful gases into the atmosphere [12].
- The recovery of metals from waste MLCCs (Multilayer Ceramic Capacitors) has been the subject of limited research. Studies have reported the recovery of metals from MLCCs through mechanical separation and hydrometallurgical processes [13-15]. Various leaching agents have been used for Ni recovery, but all have involved the use of inorganic acids such as HCl, H2SO4, and HNO3. Additionally, processes for recovering valuable metals through electrostatic separation have also been documented [13]. However, a review of the literature reveals a lack of sufficient metal recovery technologies from MLCCs and highlights the need for the development of feasible and relatively simple processes. Present research reports a novel, the experimental recovery of metals from MLCCs using simple and cost-effective leaching and precipitation agents, aiming to achieve metal recovery without environmental pollution.
- In this study, the behavior of organic acid in leaching metal ions from waste MLCC was investigated, employing organic acids that do not emit toxic gases and are readily biodegradable, thus minimizing secondary contamination. After grinding the MLCC, a leaching process was conducted using these organic acids to assess the interaction between the acids and the metals. The precipitation process, one of the hydrometallurgy processes, was used to recover metal ions from leachate. Each metal element's recovery behavior and microstructural characteristics were compared across varying concentrations of precipitating agents to elucidate the interactions between the precipitant and metal ions. A chelation process was also applied to the leached metal ions to form chelated ion solutions. To establish the optimal recovery conditions, the phases of the recovered powders were analyzed as a function of the thermal treatment temperature.
1. Introduction
- 2.1. Selective Recovery of Internal Metals from MLCCs Using a Wet Process
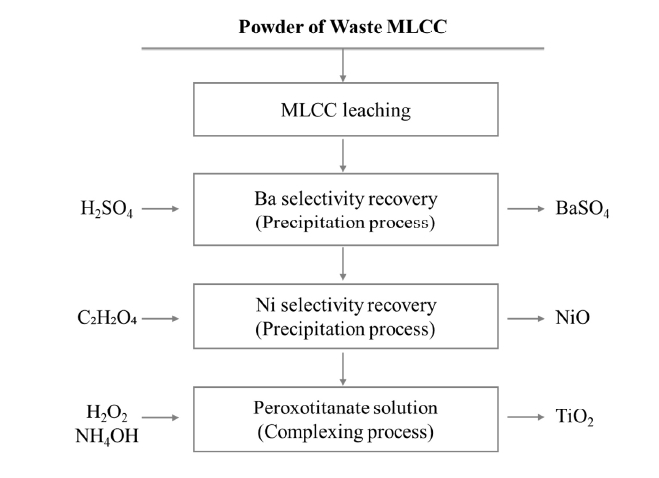
- In this study, metal recovery experiments were conducted using MLCC (Samsung Electro-Mechanics Co., Ltd, Korea), which were ground. The leaching process was performed with 1.5 M citric acid at 80 °C for 168 hours under the conditions of a solid to liquid ratio (S/L) of 33.3 g/L and 300 rpm, and then the leaching residue and the filtrate were separated. Ba from the leachate was recovered via a precipitation process using sulfuric acid (H2SO4, 95%, Daejung, Korea) at room temperature. Sulfuric acid concentrations of 0.1 M, 0.5 M, and 1 M were utilized for the precipitation. The recovery of Ni was achieved at 50°C using 0.5 M oxalic acid (C2H2O4, 99.5%, Daejung, Korea), resulting in the formation of Ni-based powder. The recovered Ni-based powder was then subjected to heat treatment at 500°C for 2 hours in an air atmosphere. Following the recovery of Ba and Ni, the residual leachate was used to recover Ti. The Ti recovery process involved using hydrogen peroxide (H2O2, 30%, Daejung, Korea) as a complexing agent, added in a 1:1 mass ratio with the residual leachate. Ammonium hydroxide (NH4OH, 35%, Daejung, Korea) was employed to adjust the pH 3.0, preparing a peroxotitanate solution at room temperature. The resulting solution was heat treated at 500°C and 800°C for 2 hours in an air atmosphere. The process flow diagram for the leaching and internal metal recovery from MLCCs in this study is illustrated in Fig. 1.
- 2.2 Characterization and Evaluation
- To analyze the phases of the crushed waste MLCC and the recovered Ba, Ni, and Ti elements, X-ray diffraction (XRD, MiniFlex600, Rigaku) was performed. The powder's surface morphology and particle size were observed with Field Emission Scanning Electron Microscopy (FE-SEM, S-4700, Hitachi). The absorbance of the peroxotitanate complex, prepared from the MLCC leachate solution, was compared using Ultraviolet-Visible Spectrophotometry (UV-Vis, Optizen POP, KLAB).
2. Experimental Procedure
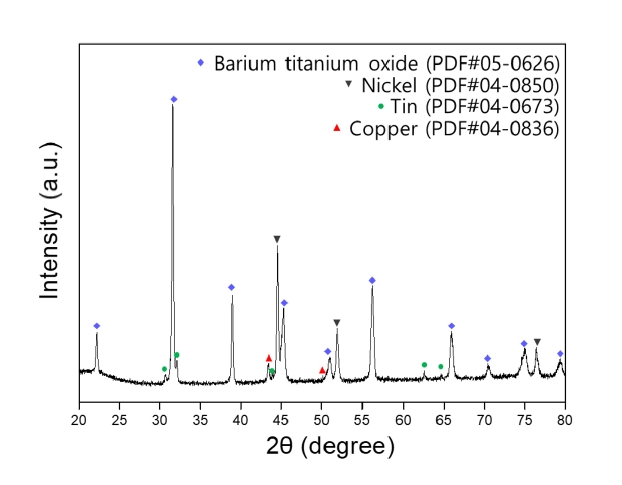
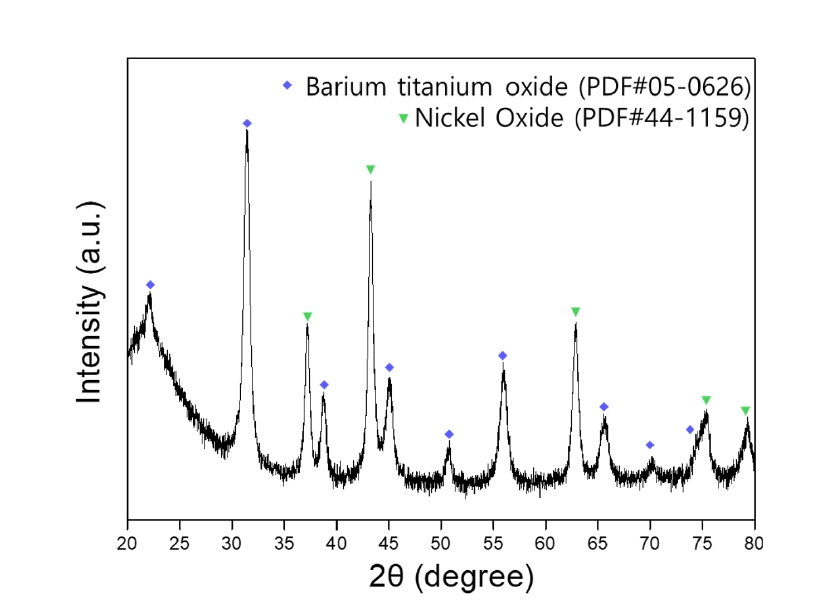
- XRD analysis was conducted to examine the phase composition of MLCC powder pulverized by the grinder. Fig. 2 shows the phase analysis results of the pulverized MLCC, confirming the presence of BaTiO3 as the dielectric material, Ni and Cu used as electrodes, and Sn used for plating [16].
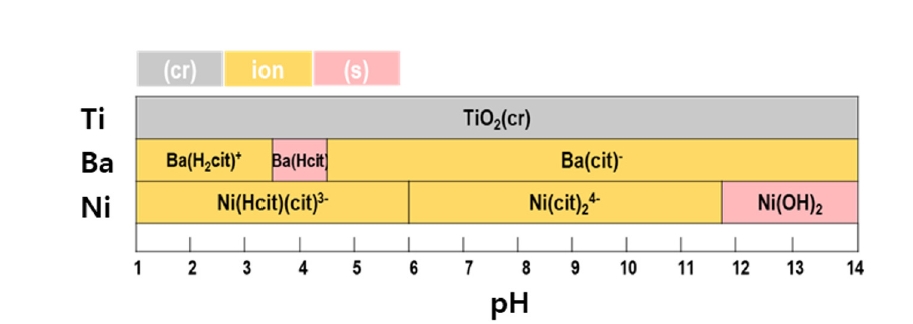
- A leaching process was conducted to recover the metals within the MLCC. Citric acid, an organic acid, was used as the leaching agent. The Pourbaix diagram was utilized to select citric acid as the leaching agent, predicting the possibility of metal leaching reactions of Ba, Ni, and Ti with citric acid depending on the pH. Fig. 3 illustrates the Pourbaix diagram for the reactions between citric acid and Ba, Ni, and Ti. The initial pH value of the leaching agent was measured at 2. After 168 hours of leaching, the pH value decreased to 1.
- The pulverized MLCC was leached in citric acid at 80°C for 168 hours, and the leached ions were identified by heat-treating the leach solution. The leached metal salt solution was subjected to heat treatment at a high temperature of 800°C, causing the solvent to evaporate. Through this thermal decomposition process, the metal ions in the solution were oxidized and subsequently recovered as powder, enabling the identification of the leached elements. The phase analysis results of the metal oxides recovered through the pyrolysis process are shown in Fig. 4, confirming the presence of BaTiO3 and NiO phases. After 168 hours of leaching, the nickel powder from the crushed MLCC was entirely leached becoming invisible. Additionally, the presence of residual nickel powder was tested using magnetism, and the lack of magnetic separation suggests that all the nickel has been fully leached.
- When the metal elements within the MLCC are leached and stirred in citric acid for an extended period, the thermodynamically unstable BaTiO3 under acidic conditions begins to leach Ba2+ ions in the low pH environment. This results in changes to the Ba/Ti ratio and the dissociation of Ba and Ti ions [17]. Additionally, citric acid, employed as the leaching agent, contains three carboxyl groups and one hydroxyl group, which offer multiple binding sites. This molecular structure imparts strong chelating properties with various metal ions, facilitating the formation of stable chelate compounds [18]. Consequently, Ti ions, dissociated from BaTiO3, are chelated, and the dissolution process is facilitated, resulting in the partial dissolution of Ti ions [19]. The following chemical equation represents the ionization process of Ba, Ti by citric acid.
- The dissolution of Ni in citric acid was confirmed through Fig. 4, illustrating the ionization process as shown in Equation (3).
- From the MLCC leachate, Ba, Ti, and Ni ions were identified as having been leached. The initial step in element recovery focused on the extraction of Ba. A precipitation process using sulfuric acid was employed to precipitate Ba ions from the leachate as a powder. Sulfate ions in sulfuric acid typically form insoluble compounds with metal ions, facilitating precipitation. Thus, by reacting Ba2+ ions in the leachate with SO42- ions from the added sulfuric acid, Barium sulfate (BaSO4) precipitates were formed, enabling the recovery of Ba from the MLCC leachate solution [20]. The reaction involved in sulfuric acid precipitation is represented by Equation (4).
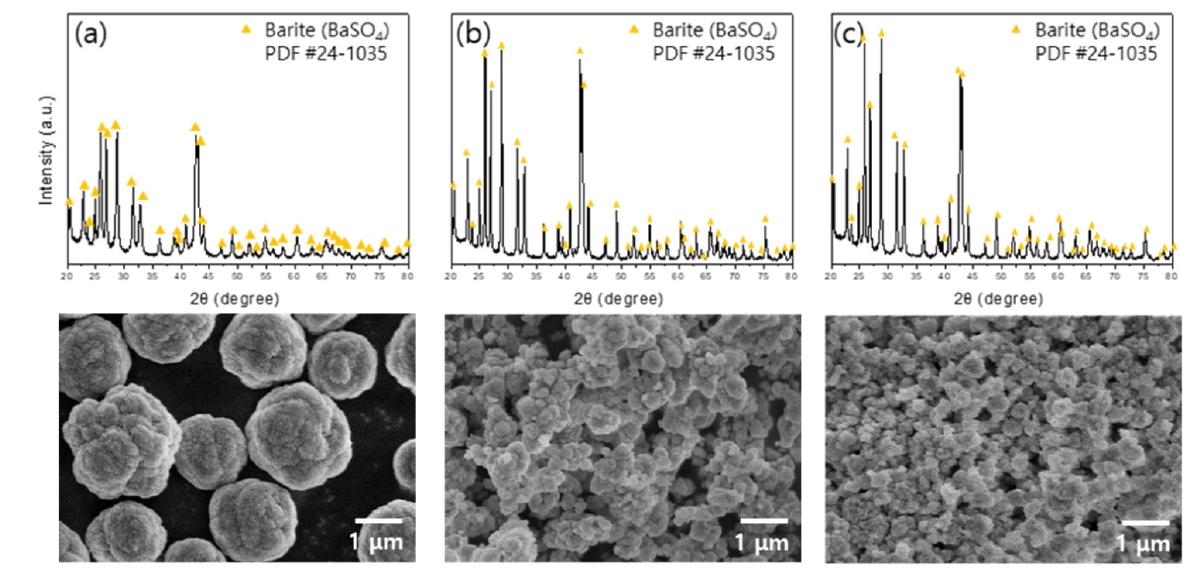
- The leachate and sulfuric acid were mixed in a 1:1 volume ratio, and the precipitation was conducted at room temperature for three hours using sulfuric acid concentrations of 0.1 M, 0.5 M, and 1 M. The crystalline phase and morphology of the precipitated powder were analyzed using XRD and FE-SEM, respectively, with the results presented in Fig. 5. The Fig. 5(a, b, c) show that the precipitated powders were confirmed to be BaSO4 particles. The precipitation yield of Ba was compared across different sulfuric acid concentrations. Approximately 84% of Ba was recovered using 0.1 M sulfuric acid, while around 92% was precipitated with 0.5 M and 1 M sulfuric acid. The lower recovery rate with 0.1 M sulfuric acid suggests incomplete precipitation of Ba within three hours. Considering the similar recoveries of 0.5 M and 1 M, 0.5 M sulfuric acid is optimal for Ba recovery. Morphological analysis indicated smaller particle sizes with higher sulfuric acid concentrations. This is likely due to the increased supersaturation in the solution, leading to rapid nucleation and limited crystal growth due to the finite amount of precipitant, resulting in smaller particles [21]. The particles precipitated with 0.1 M sulfuric acid exhibited a cauliflower-like morphology. Lower precipitant concentration leads to slower particle growth and fewer nucleation sites, causing particle aggregation as they stabilize, resulting in the growth of irregular cauliflower-shaped particles [22, 23].
- Following Ba ion recovery, Ni precipitation was undertaken using the oxalic acid precipitation method. When oxalic acid was added to the Ni ion leachate, it reacted with the oxalate from the added oxalic acid, reaching saturation. At this saturation point, Ni ions precipitated as a powder [24]. The following equation represents the precipitation reaction.
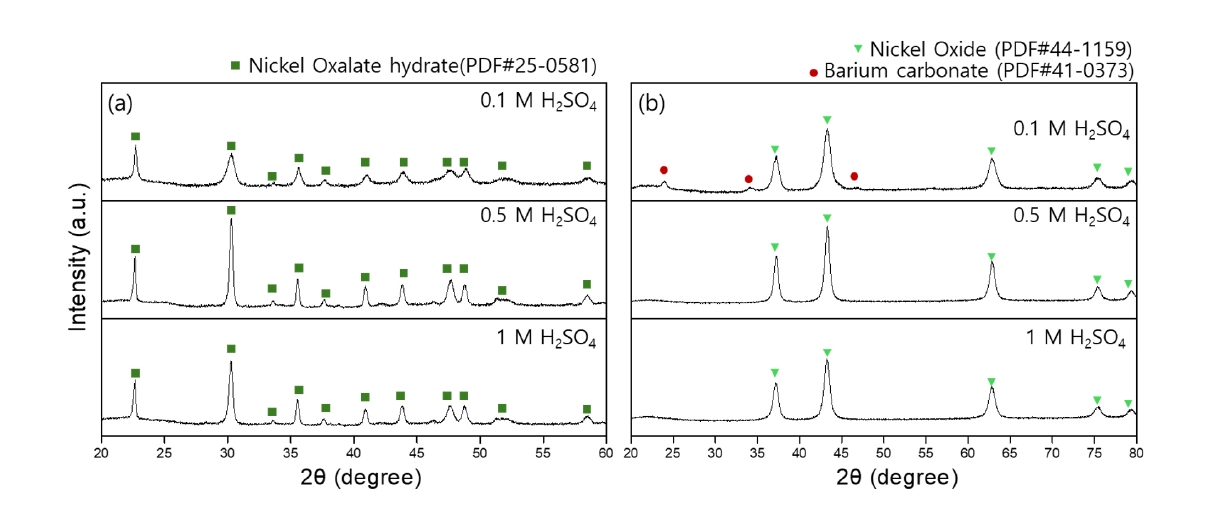
- Ni-based powder was precipitated using 0.5 M oxalic acid at 50°C. It was anticipated that the phase and morphology of the recovered Ni-based powders would vary depending on the sulfuric acid concentration (0.1 M, 0.5 M, 1 M) used for Ba recovery. Therefore, the Ni-based powders recovered at different sulfuric acid concentrations were analyzed. XRD analysis was conducted for phase analysis, and Fig. 6 presents the phase analysis results before and after heat treatment of the recovered powders. Fig. 6(a) shows the phase analysis results confirmed that the precipitated Ni-based powder was nickel oxalate hydrate (NiOx). Generally, nickel oxalate is used as a precursor for Nickel oxide (NiO), produced by heat treatment at temperatures above 450°C [25]. The reaction for forming NiO is represented by Equation (6) [24].
- Fig. 6(b) presents the phase analysis results of NiO produced from NiOx powder after heat treatment at 500°C for 2 hours. The powders recovered from the residual solutions using 0.5 M and 1 M sulfuric acid were confirmed to be NiO. However, the powder recovered from the residual solution using 0.1 M sulfuric acid exhibited not only NiO peaks but also BaSO4 peaks. The XRD analysis revealed a multiphase composition, indicating the presence of multiple crystalline phases within the powder. This suggests that residual Ba ions reacted with oxalic acid to form BaCO3, indicating that the 0.1 M sulfuric acid solution was of insufficient concentration to fully recover the Ba ions, likely due to a slower reaction rate. Phase analysis was conducted on the precipitated powders obtained after Ba recovery using 0.5 M and 1 M sulfuric acid and subsequent precipitation with 0.5 M oxalic acid. Grain size was assessed by analyzing the full width at half maximum (FWHM) of the XRD peaks. After barium recovery with 0.5 M and 1 M sulfuric acid, the grain sizes of the nickel oxalate recovered using oxalic acid were confirmed to be 29 nm and 31 nm, respectively. For the nickel oxide, the primary particle sizes were 14 nm and 15 nm, respectively.
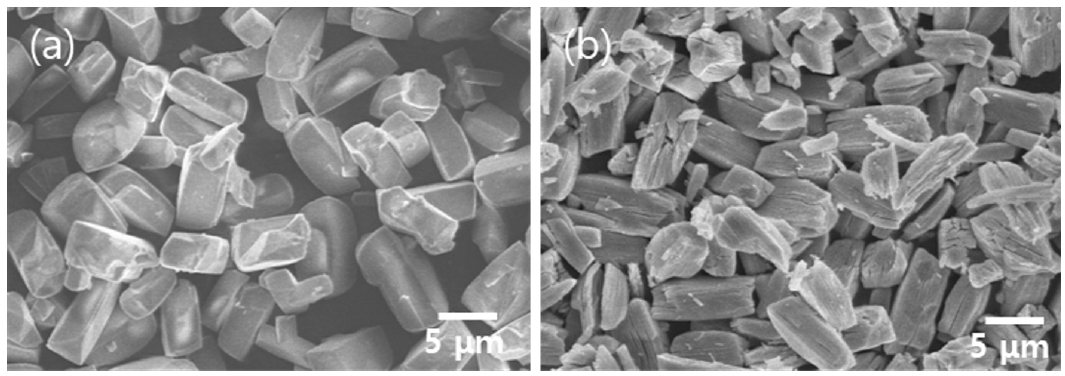
- FE-SEM analysis was conducted to examine the microstructure and particle size of the recovered Ni-based particles. Fig. 7(a) shows the FE-SEM results of NiOx recovered using 0.5 M sulfuric acid followed by 0.5 M oxalic acid, and Fig. 7(b) shows the FE-SEM results of nickel oxide. The particle sizes of NiOx and NiO, determined through image analysis, were 6.5 μm and 4.2 μm, respectively. Compared to the surface of NiOx particles, the surface of nickel oxide particles exhibited cracks and porosity. This can be explained by the decomposition of oxalate during heat treatment, which releases a large amount of CO2, resulting in the formation of cracks and pores on the surfaces and internal structure of the particles [26]. The recovery rate of nickel from the MLCC was confirmed to be 93%.
- Ba and Ni were recovered from MLCCs, followed by the preparation of a titanium complex for the final recovery of Ti. To recover titanium dioxide (TiO2), a peroxotitanate solution was prepared using hydrogen peroxide. As a transition metal, titanium forms Ti3+ or Ti4+ ions upon ionization. Ligands interact with these metal ions through coordination covalent bonds, forming coordination complexes in which the central titanium atom is bound to other atoms or molecules. This recovery process synthesized titanium complexing ions via a ligand exchange reaction between titanium ions and hydrogen peroxide [27]. Citric acid, used as the leaching agent, acted as a strong double ligand in forming transition metal ion complexes and served as a ligand exchange agent, resulting in a stable peroxotitanate solution [28]. A yellowish peroxotitanate complexing ion solution was prepared, as outlined in Equation (7).
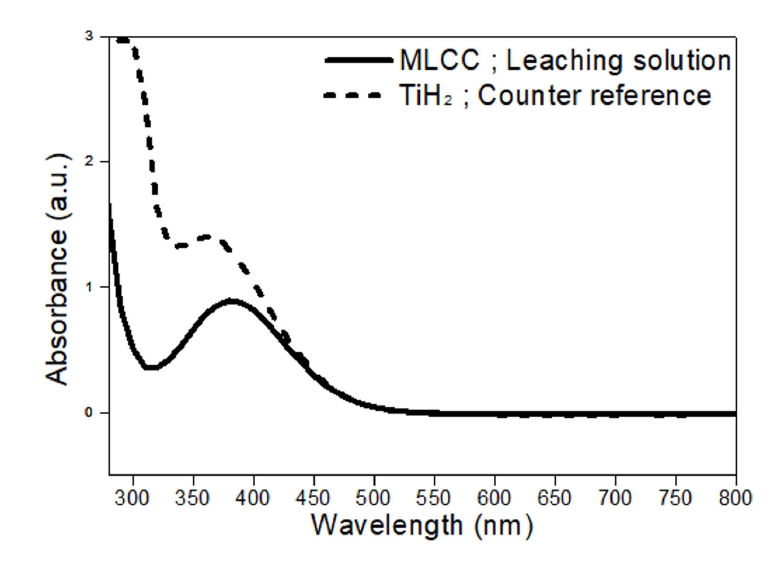
- Fig. 8 shows the absorbance analysis of the peroxotitanate complexing ion solution derived from MLCC leachate. The absorbance spectra of a complex synthesized from a titanium hydride (TiH2) precursor were analyzed for comparison. Both samples exhibited absorbance peaks in the 300-400 nm wavelength range. The similar peak profiles indicate that the complex derived from the MLCC leachate is likely peroxotitanate.
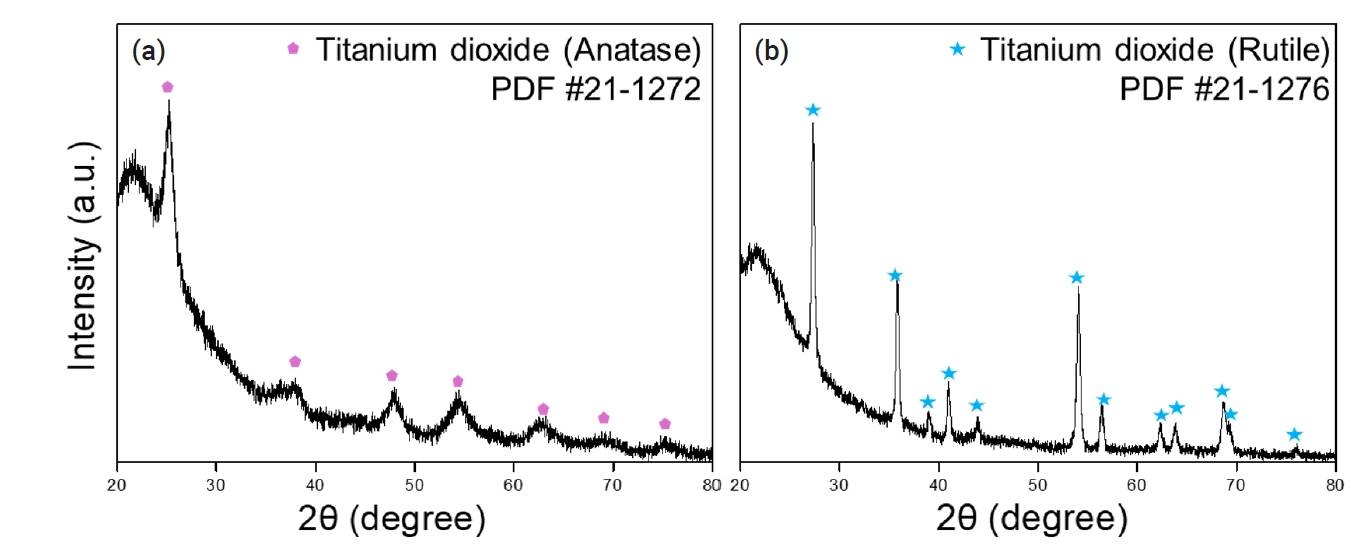
- The prepared peroxotitanate complexing ion solution was subjected to heat treatment at 500°C and 800°C for 2 hours to recover TiO2 powder. Fig. 9 presents the XRD analysis results, demonstrating the presence of both Anatase and Rutile phases of TiO2. Typically, Anatase is synthesized at 500°C, while Rutile forms at temperatures above 800°C [28-30]. The recovered TiO2 was confirmed to undergo phase transitions to Anatase at 500°C and Rutile at 800°C.
3. Results and Discussion
- In this study, citric acid, an organic acid, was employed for the leaching process to recover Ba, Ni, and Ti elements from MLCCs. Precipitation processes using sulfuric acid and oxalic acid were conducted to recover BaSO4 and NiOx, respectively. The morphology of the recovered BaSO4 was investigated as a function of sulfuric acid concentration, and the phase and morphology changes of the Ni-based powders were examined. It was observed that lower concentrations of sulfuric acid did not result in complete recovery of Ba, leading to the presence of Ba in the Ni-based powders during Ni recovery. This finding enabled the determination of the optimal sulfuric acid concentration for Ba recovery. NiOx, recovered via the oxalic acid precipitation method, was heat treated to produce NiO powder. The morphological changes of NiO particles due to heat treatment were analyzed. Ultimately, 93% of the nickel powder was recovered from the MLCC. A peroxotitanate complex ion solution was prepared for Ti recovery using hydrogen peroxide. The hydrogen peroxide facilitated ligand exchange reactions among Ti ions, and citric acid, used as the leaching agent, acted as a ligand substituent to form a stable peroxotitanate solution. This solution was then converted into powder through heat treatment, allowing the recovery of TiO2 powder in the anatase and rutile phases by adjusting the heat treatment temperature. The recovered BaSO4, NiO, and TiO2 are anticipated to have applications in the medical, electronics, and chemical industries, contributing to resource conservation and environmental and cost savings. Moreover, the metal recovery process used in this study is expected to apply to the recycling processes of various waste electronic products, such as PCBs and batteries, in addition to MLCCs.
4. Conclusion
-
Conflict of Interest Declaration
The authors declare no competing financial interests or personal relationships.
-
Author Information and Contribution
H. I. Shin: Graduated student, analyzed the data, experiment, wrote the initial manuscript. K. J. Lee: Professor, conceived and designed the study. All authors approved the final version of the manuscript.
-
Acknowledgement
This work was supported by Korea Environment Industry & Technology Institute (KEITI) through R&D Project of recycling development for future waste resources program, funded by Korea Ministry of Environment (MOE) (2022003500003) and the National Research Foundation of Korea(NRF) grant funded by the Korea government(MSIT) (No. 2022R1A2C1007909).
Article information
- 1. M. A. Rabah, F. E. Farghaly and M. A. Abd-El: Motaleb: Waste Manag., 28 (2008) 1159.Article
- 2. H. L. An, L. S. Kang and C. G. Lee: Resources Recycling, 26 (2017) 4.
- 3. K. T. Hong, T. H. Lee, J. M. Suh, S. H. Yoon and H. W. Jang: J. Mater. Chem. C, 7 (2019) 9782.Article
- 4. Y. Kim, J. C. Lee, B. S. Ki, M. S. Kim and J. K. Jeong: Hydrometallurgy, 86 (2007) 89.Article
- 5. Y. Liu, Q. Song, L. Zhang and Z. Xu: J Clean Prod., 290 (2021) 125650.Article
- 6. M. D. Najafi, S. K. Eshkalak, B. Amiri, H. R. Naderi, E. Kowsari, A. Chinnappan and S. Ramakrishna: Mater Today Chem., 23 (2022) 100633.
- 7. Y. G. Kim: J. Powder Mater., 11 (2004) 265.Article
- 8. T. P. Mokoena, H. C. Swart and D. E. Motaung: J. Alloys, 805 (2019) 267.Article
- 9. H. C. Jung, G. H. Kim, H. S. Hong and D. W. Kim: J. Powder Mater., 17 (2010) 175.Article
- 10. X. Chen, Y. Chen, T. Zhou, D. Liu, H. Gu and S. Fan: Waste Manage, 38 (2015) 349.Article
- 11. R. Golmohammadzadeh, F. Faraji and F. Rashchi: Resour. Conserv. Recycl. Adv., 136 (2018) 418.Article
- 12. L. Li, J. B. Dunn, X. X. Zhang, L. Gaines, R. J. Chen, F. Wu and K. Amine: J. Power Sources, 233 (2013) 180.Article
- 13. B. Niu and Z. Xu: Sustainable Mater. Technol., 21 (2019) e00101.Article
- 14. D. Fontana, M. Pietrantonio, S. Pucciarmati, GN. Torelli, C. Bonomi and F Masi: J. Mater. Cycles Waste Manage, 20 (2018) 1199.ArticlePDF
- 15. G. Prabaharan, SP. Barik and B. Kumar: Waste Manage, 52 (2016) 302.Article
- 16. K. Hong, T. H. Lee, J. M. Suh, S. H. Yoon and H. W. Jang: J. Mater. Chem. C. Mater., 7 (2019) 9782.Article
- 17. S. S. Tripathy and A. M. Raichur: J. Exp. Nanosci., 6 (2011) 127.Article
- 18. W. Astuti, T. Hirajima, K. Sasaki and N. Okibe: Miner Eng., 85 (2016) 1.Article
- 19. W. Jonglertjunya and T. Rubcumintara: Asia Pac. J. Chem. Eng., 8 (2013) 323.Article
- 20. A. A. Öncül, K. Sundmacher, A. Seidel-Morgenstern and D. Thévenin: Chem Eng Sci., 61 (2006) 652.Article
- 21. J. Y. Park, D. W. Jeong, K. T. Park, T. S. Kim, Y. H. Choa and B. S. Kim: J. Nanosci. Nanotechnol., 16 (2016) 10630.Article
- 22. M. Khalil, J. Yu, N. Liu and R. L. Lee: J. Nanopart Res., 16 (2014) 1.Article
- 23. J. Kontrec, N. Tomašić, N. Matijaković Mlinarić, D. Kralj and B. Njegić Džakula: Crystals, 11 (2021) 1075.Article
- 24. S. Surianti, K. C. Wanta, W. Astuti, F. R. Mufakhir, I. Perdana and P. H. Petrus: J. Min. Sci., 58 (2022) 476.ArticlePDF
- 25. P. W. M Jacobs and A. R. T. Kureishy: Phys Chem Chem Phys., 58 (1962) 551.Article
- 26. K. K. Sahu, R. K. Sahoo, L. D. Beshra and M. Mohapatra: Ionics, 27 (2021) 819.ArticlePDF
- 27. Y. Kang, J. Park and K. J. Lee: J. Adhes. Sci. Technol., 31 (2017) 2571.Article
- 28. K. Tomita, V. Petrykin, M. Kobayashi, M. Shiro, M. Yoshimura and M. Kakihana: Angew. Chem. Int. Ed. Engl., 45 (2006) 2378.Article
- 29. D. A. H. Hanaor and C. C. Sorrell: J. Mater. Sci., 46 (2011) 855.ArticlePDF
- 30. Z. Xiong, H. Wu, L. Zhang, Y. Gu and X. S. Zhao: J. Mater. Chem. A. Mater. A, 2 (2014) 9291.Article
References
Figure & Data
References
Citations

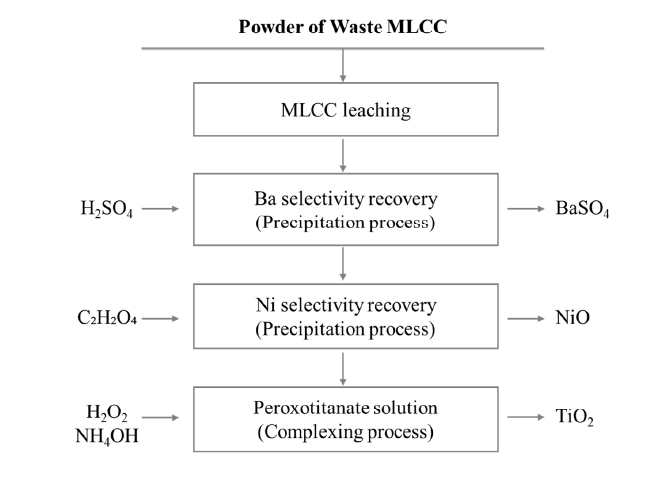
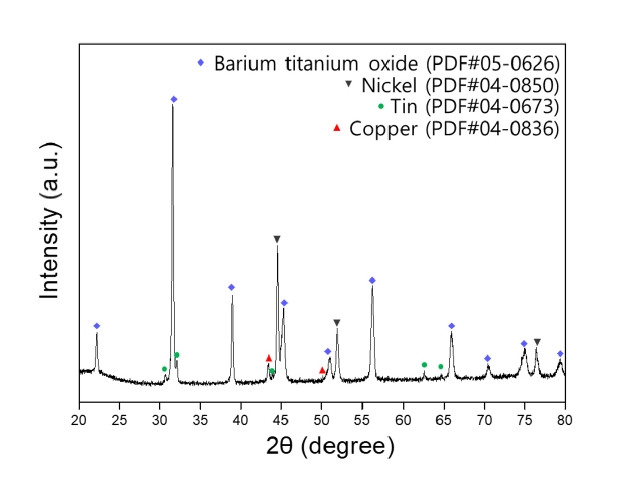
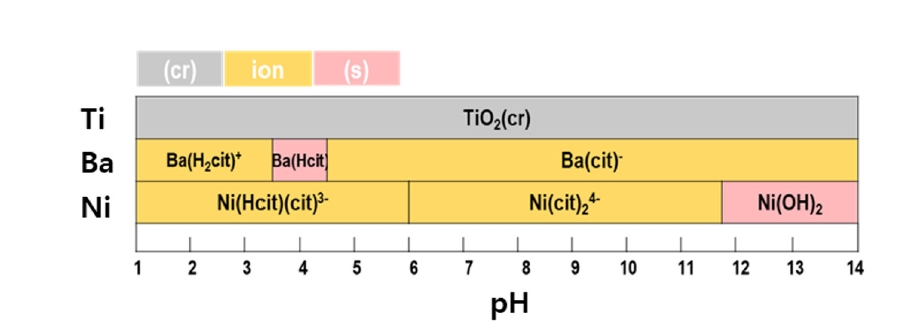
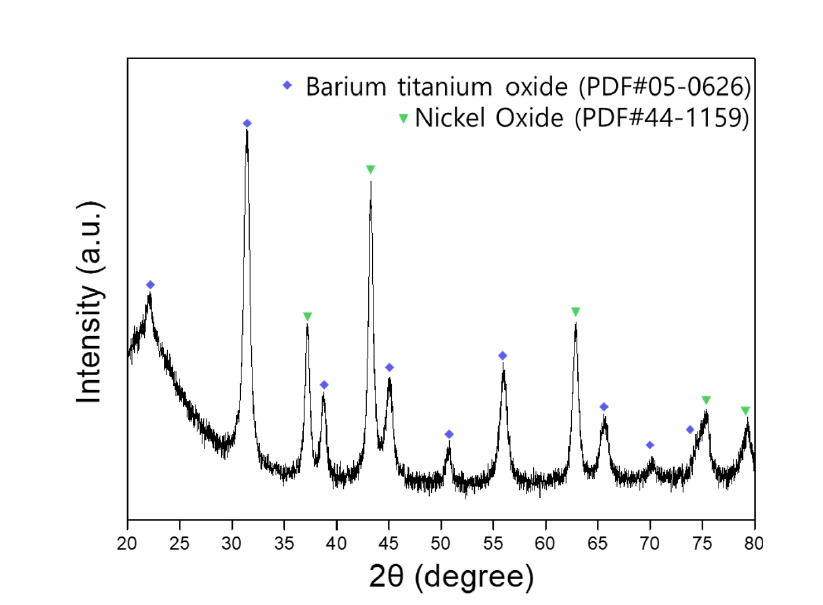
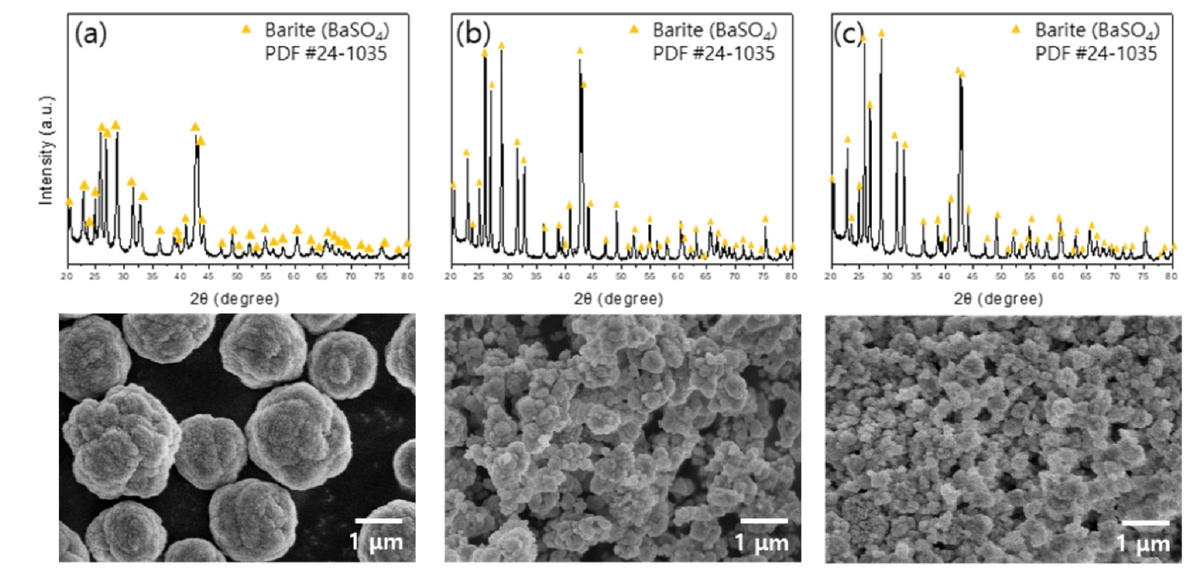
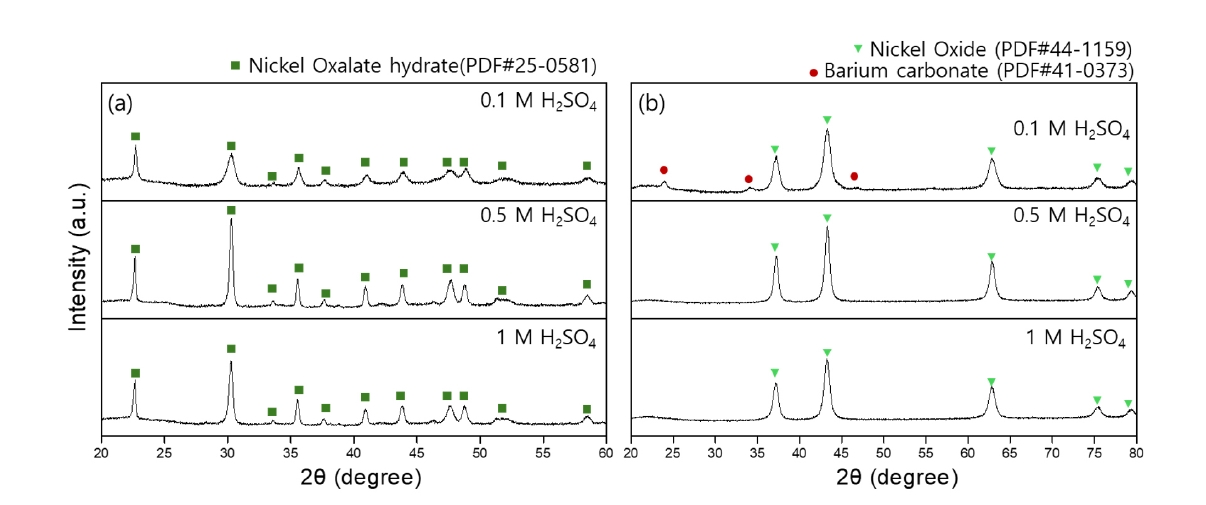
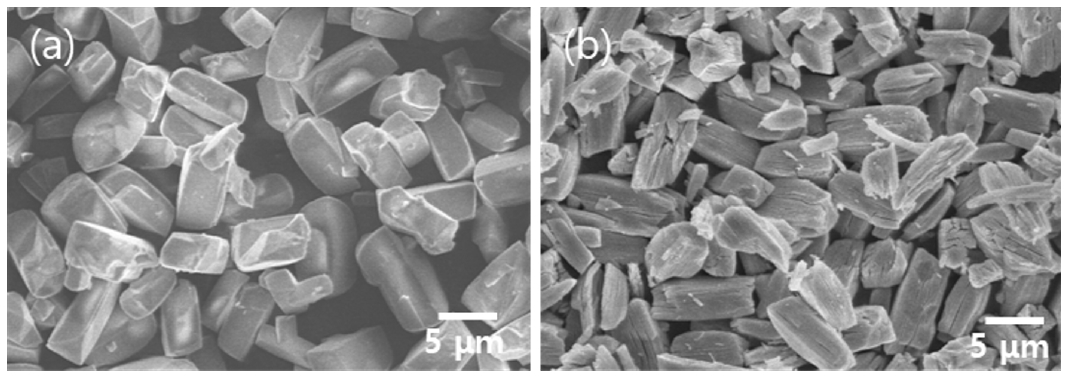
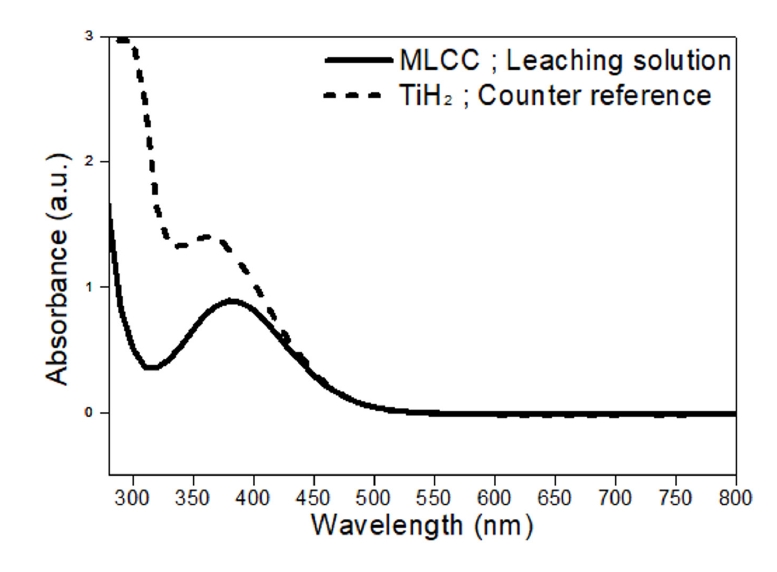
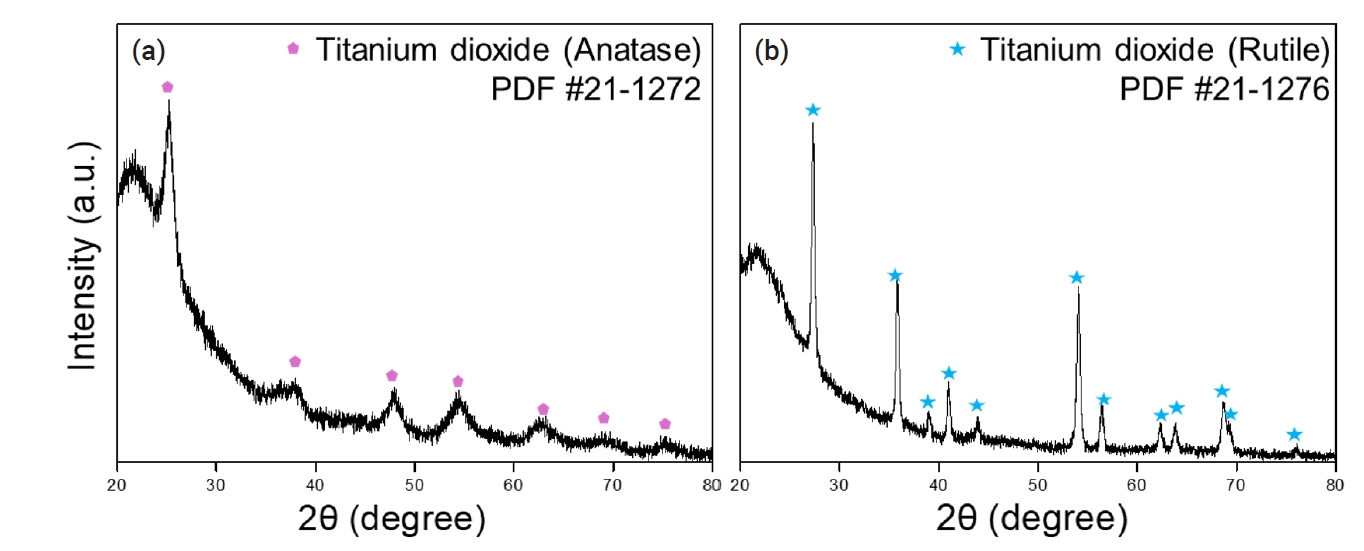
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
Fig. 7.
Fig. 8.
Fig. 9.
TOP
 KPMI
KPMI

 ePub Link
ePub Link Cite this Article
Cite this Article









