Articles
- Page Path
- HOME > J Powder Mater > Volume 32(5); 2025 > Article
-
Research Article
- Morphological Control and Surface Modification Characteristics of Nickel Oxalate Synthesized via Oxalic Acid Precipitation
- Eunbi Park1, Jongwon Bae1, Sera Kang1, Minsu Kang1, Suseong Lee2, Kun-Jae Lee1,2,*
-
Journal of Powder Materials 2025;32(5):375-382.
DOI: https://doi.org/10.4150/jpm.2025.00248
Published online: October 31, 2025
1Department of Hydrogen Energy, Dankook University, Cheonan 31116, Republic of Korea
2Department of Energy Engineering, Dankook University, Cheonan 31116, Republic of Korea
- *Corresponding author: Kun-Jae Lee E-mail: kjlee@dankook.ac.kr
© The Korean Powder Metallurgy & Materials Institute
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,085 Views
- 21 Download
Abstract
- Nickel is widely used in industrial fields such as electrocatalysis and energy storage devices. Although micron-sized nickel particles exhibit excellent mechanical durability, their low specific surface area limits their reactivity. We modified the surface of micron-sized nickel particles with nanostructured nickel oxalate and investigated the effects of the solvent dielectric constant, surfactant, and thermal treatment atmosphere on the resulting particle morphology and phase transformation. Rietveld refinement analysis confirmed that changes in the solvent dielectric constant led to increased or diminished crystallinity of specific planes in nickel oxalate, resulting in diffraction patterns distinct from standard JCPDS data. These structural changes were also found to influence the morphology of the synthesized nickel oxalate. The results demonstrate that nickel oxalate serves as an effective precursor for producing Ni and NiO phases, and shape control of the final product can increase the surface reactivity of micron-sized nickel materials.
-
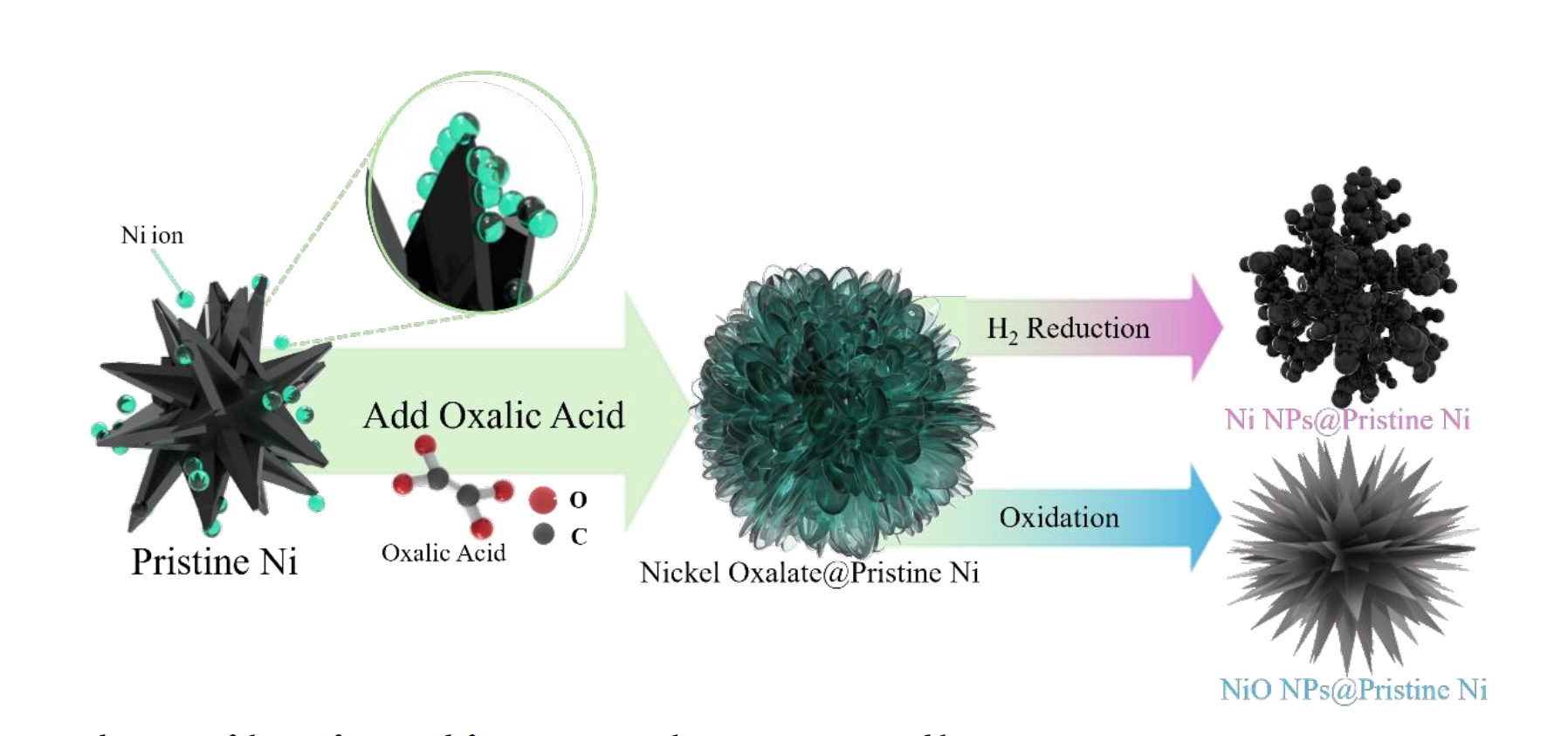
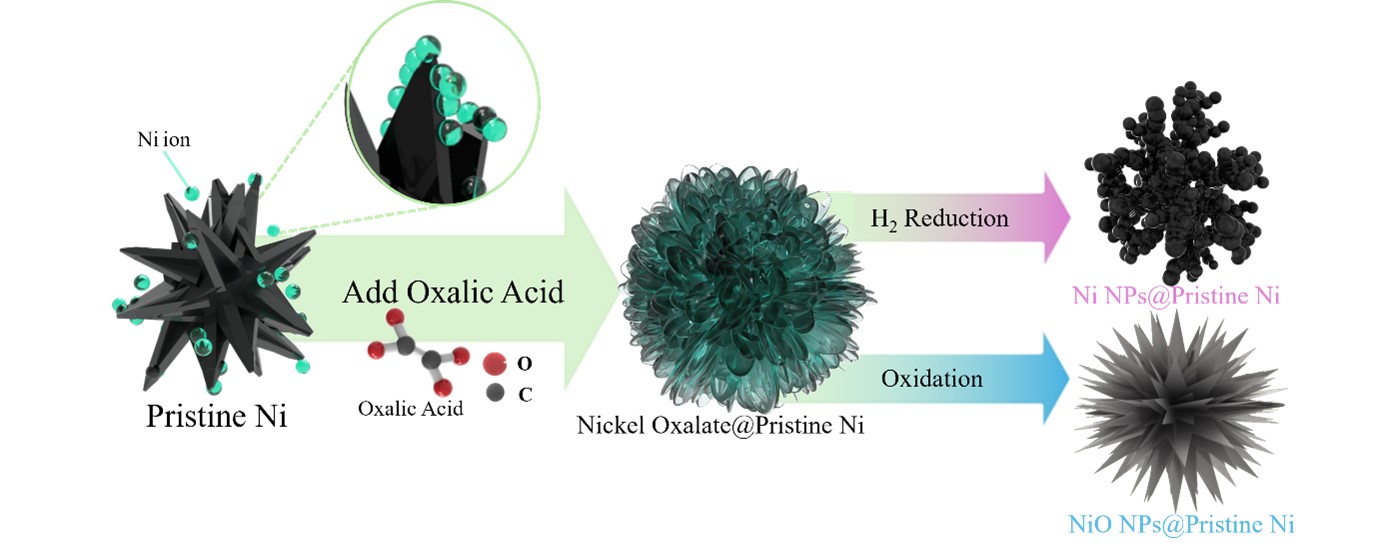
- Nickel oxalate–mediated surface modification enables controlled growth and phase transformation of nanoscale shells on micron-sized nickel particles. Morphology and phase are tailored via solvent dielectric constant and heat treatment, preserving substrate properties while enhancing surface area and reactivity.
Graphical abstract
- Nickel (Ni), a transition metal, exhibits diverse electronic structures and chemical bonding characteristics, which enable its applications in various fields, such as magnetic materials, catalysts, and energy storage devices [1, 2]. Notably, the demand for nickel has been continuously increasing alongside the growth of the electronics industry, making research on material development technologies for high-performance nickel-based materials essential [3].
- Nickel-based materials can be synthesized via various methods, including hydrothermal synthesis [4, 5], solvothermal synthesis [6], electrochemical deposition [7], high-energy ball milling [8], and thermal decomposition [9]. However, these processes often require high-temperature and high-pressure conditions, long reaction times, and precise equipment, which impose limitations on process complexity and morphological control.
- In contrast, the heat treatment-based synthesis using nickel oxalate (NiC₂O₄•xH₂O) as a precursor offers relatively straightforward reaction conditions, enabling the conversion to nickel or nickel oxide-based materials. It also allows for morphological retention and control during the crystal growth process. Notably, nickel oxalate can be selectively transformed into nickel, nickel oxide, or nickel nitride, depending on the heat treatment conditions [10]. Furthermore, phase transitions induced by thermal decomposition, doping, or electrochemical reactions facilitate post-treatment processes such as reconstruction, enhancing its versatility as a precursor [11-15].
- Among nickel oxalate synthesis methods, wet precipitation-based approaches proceed at relatively low temperatures and provide tunability of crystal morphology and phase by controlling various parameters such as precursor concentration, solvent, additives, and pH [16-20].
- Nickel-based nanomaterials offer high specific surface area and catalytic reactivity but generally suffer from low thermal and chemical stability, leading to vulnerability to delamination or oxidation under external conditions. Conversely, coarser micron-sized materials exhibit superior mechanical strength and thermal stability, but are hindered by their low specific surface area, which limits their catalytic and electrochemical activity. Therefore, surface modification techniques that improve surface reactivity while maintaining the stability of coarse particles are highly desirable [21, 22].
- In this study, nickel oxalate was precipitated onto the surface of commercial nickel powder to form a composite structure, followed by reductive heat treatment to induce transformation into Ni/NiO composite phases. We particularly investigated the effect of solvent dielectric properties on the crystal growth and morphology of nickel oxalate and analyzed the corresponding surface modification characteristics. This work aims to contribute a fundamental understanding of the crystal growth mechanism and reductive reactivity of nickel oxalate precursors, providing key insights for controlled synthesis and surface modification technology development of nickel-based materials.
1. Introduction
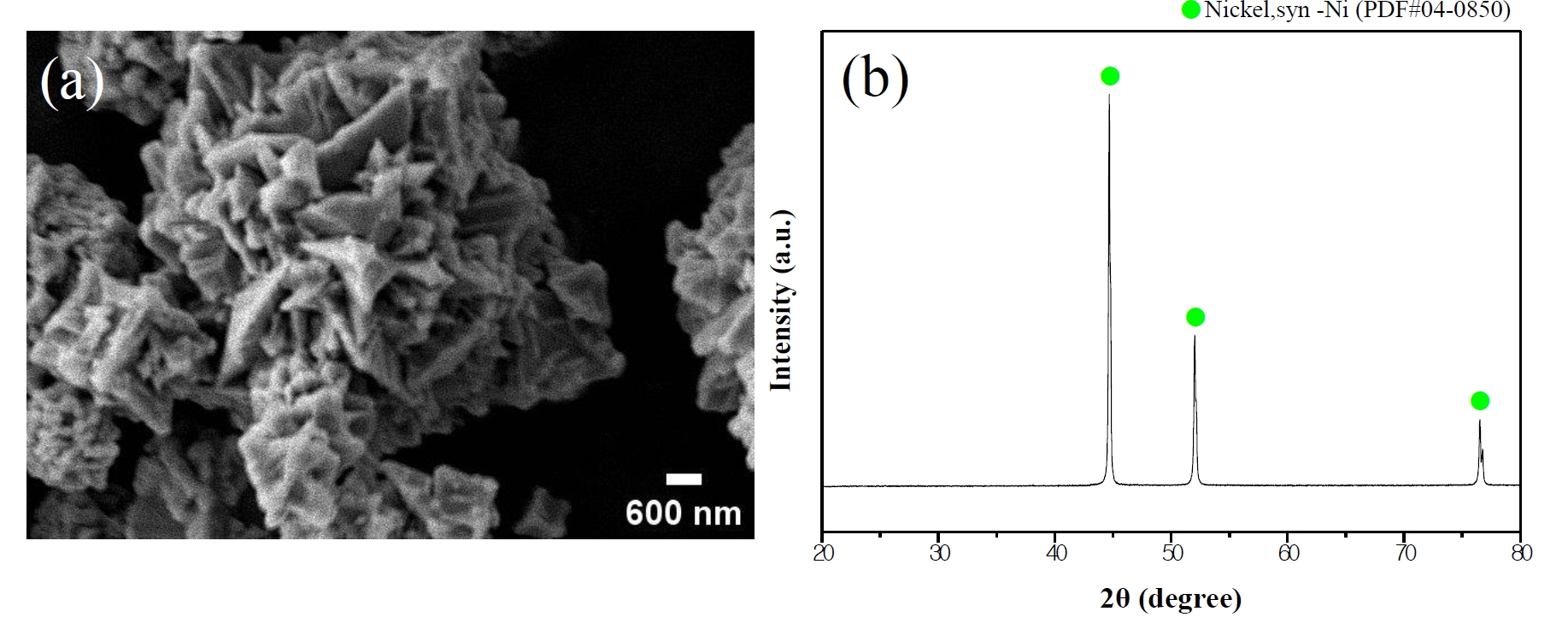
- All reagents used in this experiment were employed without additional purification. Nickel (II) chloride hexahydrate (NiCl₂•6H₂O, DAEJUNG, 96%) was used as the nickel precursor, and oxalic acid anhydrous (DAEJUNG, 98.5%) served as the precipitating agent. Deionized water and methyl alcohol (DAEJUNG, 99.5%) were used as solvents. Polyacrylic acid solution (PAA, Mw 8000–12000, FUJIFILM Wako Pure Chemical Corporation, 25%) was utilized as an additive for surface control and dispersion stabilization. Commercial nickel powder (APS 2.2–3.0 µm, Alfa Aesar, 99.9%) was employed as the substrate for surface modification. The morphology and phase of the commercial nickel powder are shown in Fig. 1.
- Nickel oxalate (NiC₂O₄•xH₂O) was synthesized via a solution precipitation method. First, 0.9 g of nickel II) chloride hexahydrate was fully dissolved in 20 mL of a mixed solvent, followed by the addition of oxalic acid anhydrous in a 1:1 molar ratio relative to Ni²⁺ ions. The resulting solution was stirred at room temperature for 24 hours to induce precipitation [23]. The obtained precipitate was sequentially washed twice with deionized water and then with ethanol. The final powder was allowed to dry naturally at room temperature and was used as the nickel oxalate precursor.
- To modify the surface and control the morphology of commercial nickel particles, nickel oxalate was directly precipitated onto their surfaces. Methyl alcohol was used as the solvent for the precipitation reaction, and polyacrylic acid (PAA) was added as an additive to enhance surface control and dispersion stability. The mixed solution was reacted at room temperature (~25 °C) for 24 hours, promoting direct deposition of nickel oxalate on the nickel surface.
- A thermal treatment process was conducted to selectively convert the nickel oxalate precipitated on the commercial nickel particle surfaces into metallic nickel or oxide phases. To remove organic components and induce reduction, heat treatment was performed at 400 °C for 1 hour under both reducing atmosphere (3% H₂ - 97% Ar) and ambient atmosphere conditions.
- Phase analysis of the precipitated nickel oxalate powder was performed using X-ray diffraction (XRD) (MiniFlex600, Rigaku). Rietveld refinement of the diffraction patterns was conducted with Profex software (based on BGMN) to evaluate the crystal structure and phase fractions quantitatively. Additionally, phase changes in the commercial nickel powder surface modified by nickel oxalate via solution precipitation were confirmed through XRD analysis. Particle morphology and size analysis of both the precipitated nickel oxalate and the surface-modified nickel powder were carried out using field emission scanning electron microscopy (FE-SEM, S-4700, Hitachi).
2. Experimental section
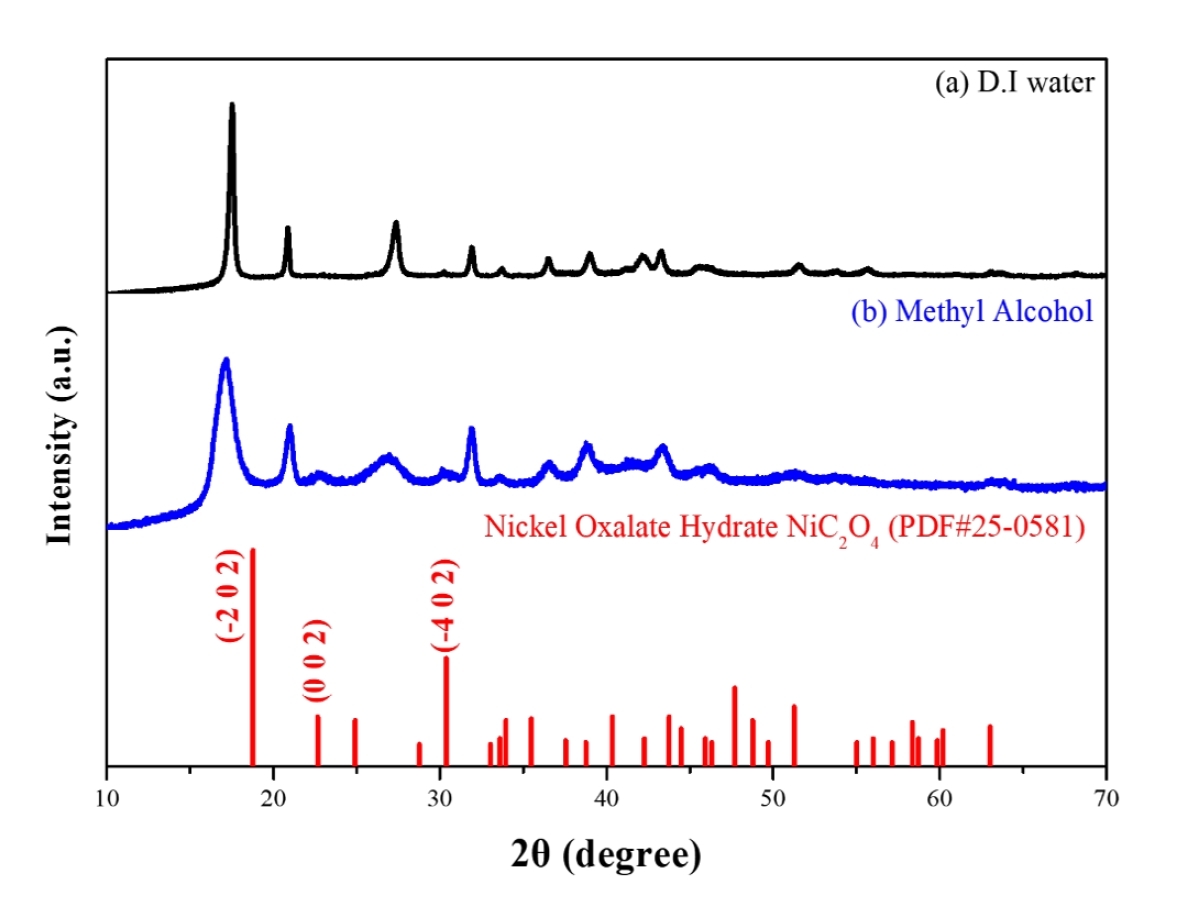
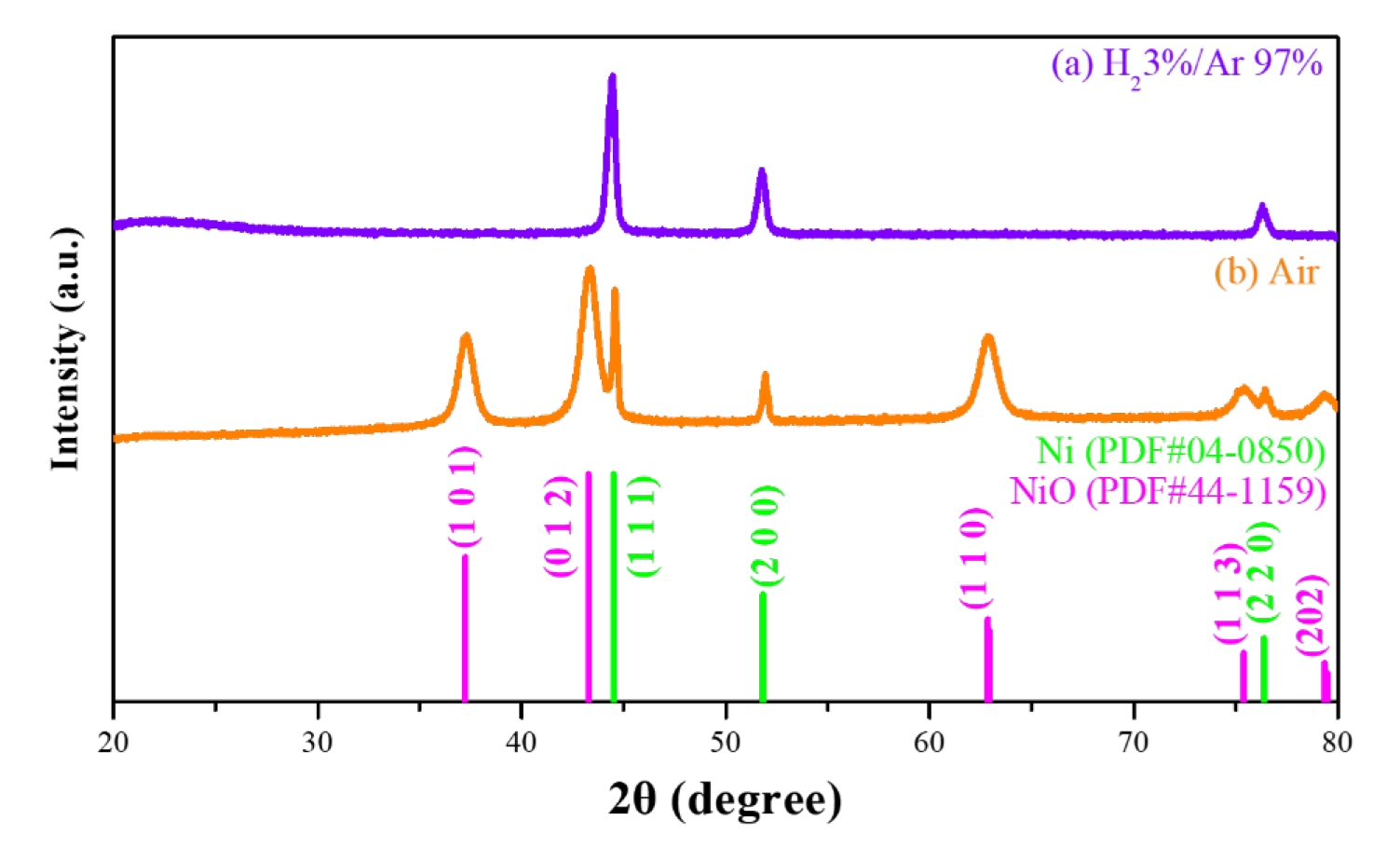
- To analyze the precipitation behavior of nickel oxalate as a function of solvent properties, nickel precursors were precipitated into nickel oxalate using oxalic acid under different solvent conditions. Fig. 2 presents the X-ray diffraction (XRD) phase analysis of the nickel oxalate precipitates obtained under varying solvent environments. The crystallization behavior and structural changes of nickel oxalate were investigated with variations in solvent dielectric constants.
- When precipitation was conducted in deionized water, a monoclinic nickel oxalate hydrate phase was observed. In contrast, precipitation in methyl alcohol resulted in a nickel oxalate hydrate phase with a crystal structure that differed from the standard JCPDS data. Distinct changes in relative diffraction intensities at specific crystal planes were evident depending on the solvent type. Particularly, when methyl alcohol was used as the solvent, a decrease in the relative diffraction intensities at the (-2 0 2), (0 0 2), and (-4 0 2) planes, as well as the disappearance of some peaks, were observed. These changes are interpreted as anisotropic crystal growth driven by high surface energies on these specific facets.
- The crystallinity of the synthesized nickel oxalate strongly depends on the solvent’s dielectric constant. Samples synthesized in low-dielectric-constant solvents, such as methyl alcohol, exhibited relatively lower crystallinity. This reduction in crystallinity is attributed to increased disorder during crystal growth caused by restricted ion dissociation and mobility. Such decreased crystallinity is expected to increase thermal instability during subsequent reduction heat treatments to metallic nickel, thereby facilitating faster reduction reactions and phase transitions.
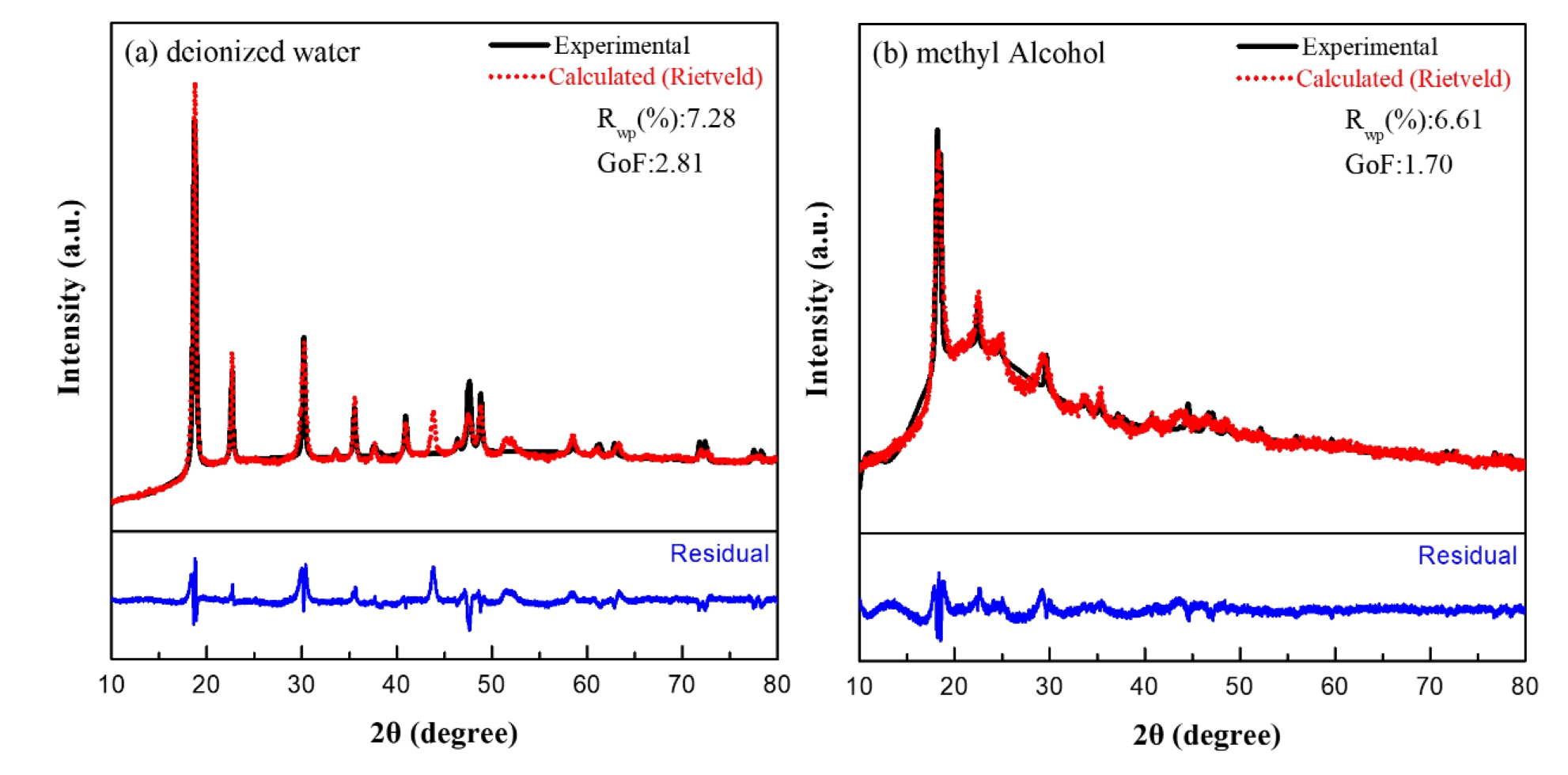
- Rietveld refinement analysis was also performed to assess the phase and crystal structure quantitatively. The Rwp value derived from the refinement represents the weighted profile R-factor, which quantifies the residual difference between the experimental diffraction pattern and the calculated theoretical pattern based on statistical weighting. This value is converted into the Goodness of Fit (GoF) index by dividing it by the statistically expected minimum error, Rexp, which provides a measure of how well the proposed structural model describes the experimental data. Fig. 3 shows the Rietveld refinement results for nickel oxalate synthesized in different solvents. In deionized water, the Rwp and GoF values were 7.28 and 2.81, respectively, whereas in methyl alcohol conditions, they yielded lower values of 6.61 and 1.70. Despite the high crystallinity indicated by sharp diffraction peaks in samples synthesized in deionized water, the relatively higher GoF is likely due to the model’s sensitivity to minor deviations in lattice parameters or atomic positions inherent in high-crystallinity specimens. These subtle mismatches increase error contributions in the refinement, resulting in a higher overall GoF.
- According to Rietveld refinement guidelines [24], in highly crystalline samples, the narrow full width at half maximum (FWHM) causes even minor discrepancies to contribute significantly to the residuals, thereby leading to a sensitive increase in the GoF value. Consequently, a GoF greater than 2 cannot be unequivocally regarded as indicative of an improper structural model. In contrast, in the case of broadened peaks, the mismatch between the observed and calculated patterns becomes less pronounced, which may result in the interpretation of relatively lower GoF values.
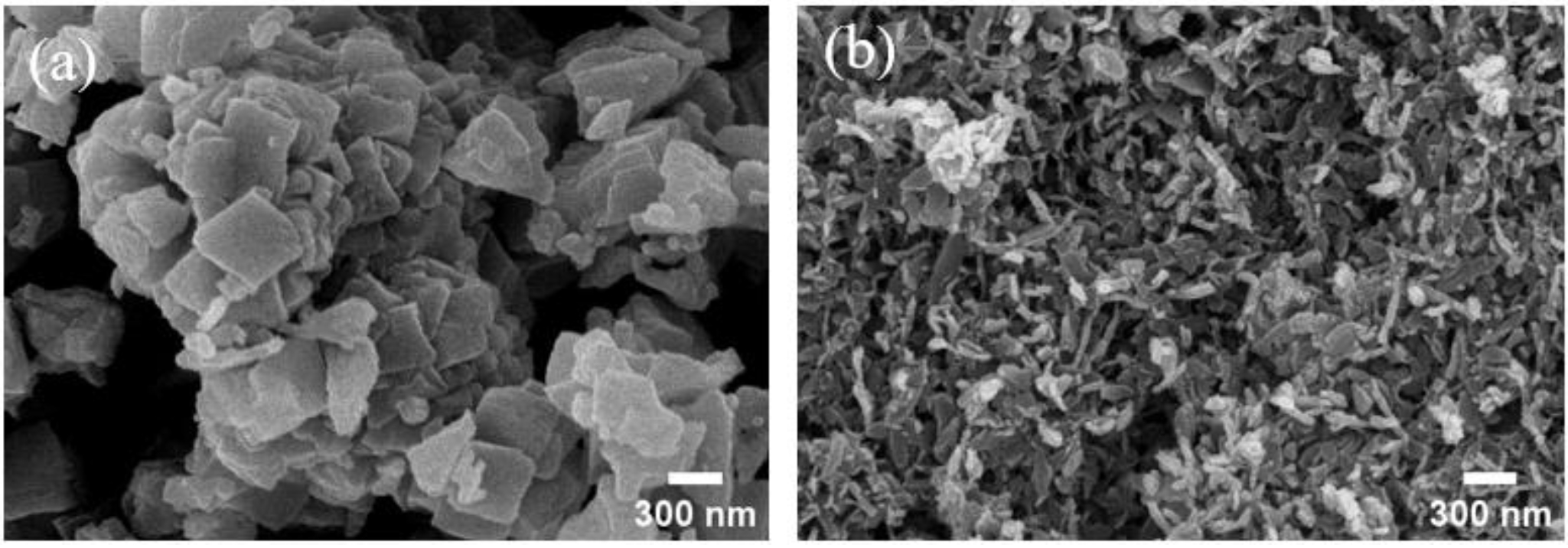
- Variations in crystal structure and particle morphology as a function of solvent properties are attributed primarily to changes in ionic mobility governed by the dielectric constant of the solvent [25]. In high dielectric constant solvents, Ni²⁺ ions exhibit increased freedom, which modifies nucleation and particle growth mechanisms via self-complexation. Fig. 4 presents field emission scanning electron microscopy (FE-SEM) images of nickel oxalate precipitated under different solvents. In solvents with low dielectric constant, particles predominantly exhibited high aspect ratio flake-like morphologies, whereas solvents with higher dielectric constants resulted in more uniform and densely packed structures. These observations suggest that solvent dielectric properties are a critical factor influencing the directionality of crystal growth and the nucleation mechanism in the precipitation process.
- To enhance the specific surface area and control the morphology of commercial nickel powder, precipitation reactions were conducted to control the shape of nickel oxalate based on the characteristics of the solvent. Additionally, polyacrylic acid (PAA) was introduced to improve dispersion stability and regulate crystal growth simultaneously. PAA acts as a capping agent by adsorbing onto the commercial nickel surface through its abundant carboxyl groups (-COOH), thereby suppressing particle agglomeration and selectively binding to specific crystal facets to direct crystal growth orientation.
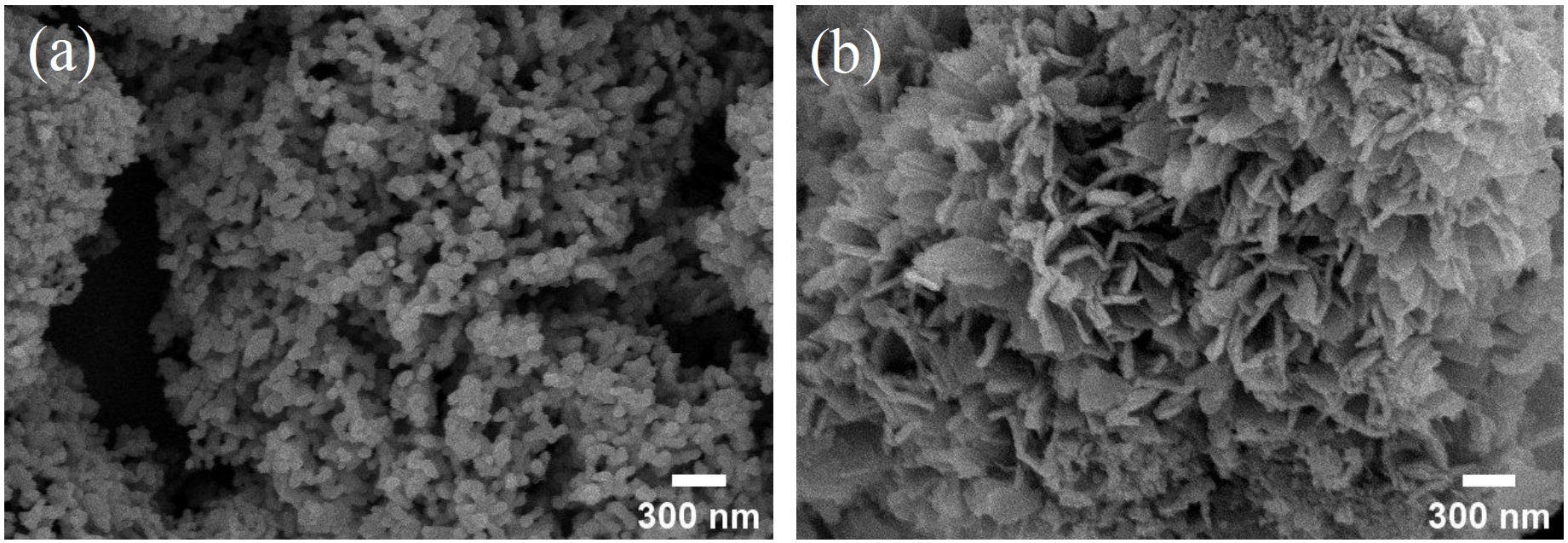
- Fig. 5 shows the FE-SEM images of the surface-modified commercial nickel powder. In the absence of PAA, nickel oxalate precipitates exhibited flake-like morphologies without developing into distinctive flower-like structures. However, with the addition of PAA, nickel oxalate precipitated on the commercial nickel surface developed into hierarchical flower-like architectures composed of flake-like units, indicating successful surface modification.
- Subsequently, the modified nickel powders were subjected to heat treatment under both reducing atmosphere (3% H₂ -97% Ar) and ambient conditions to remove organic components and induce phase transformation of the precursors. This treatment enabled selective conversion into metallic nickel or nickel oxide phases.
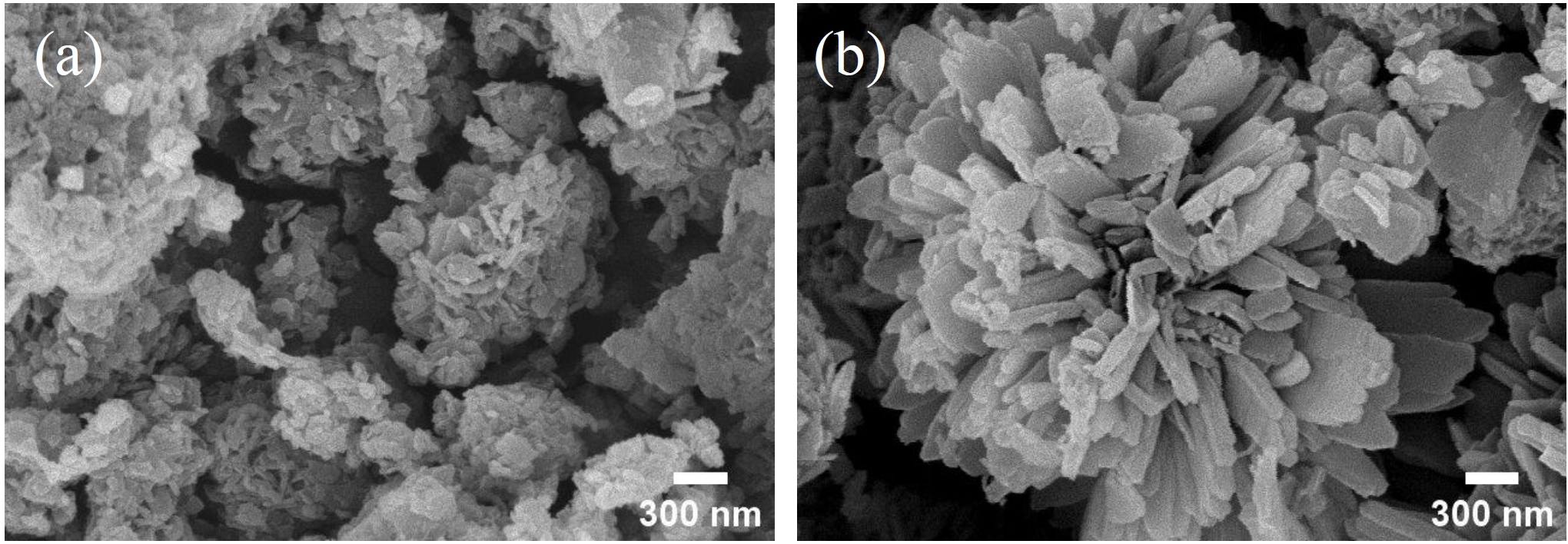
- To investigate the organic decomposition, phase transformation, and morphological control of the synthesized nickel oxalate, heat treatments were performed at 400 °C under both reducing atmosphere (3% H₂ - 97% Ar) and ambient air conditions [26]. The resulting crystal phases and morphologies of the nickel-based materials were subsequently analyzed. As shown in Fig. 6, X-ray diffraction (XRD) analysis after heat treatment revealed that the nickel oxalate was entirely reduced to a predominantly single-phase metallic nickel in the hydrogen atmosphere. Under ambient atmosphere, however, the nickel oxalate transformed into nickel oxide while the metallic nickel particle remained unoxidized, resulting in the formation of a nickel oxide coating on metallic nickel particle. The morphological evolution of the nickel oxalate under each heat treatment condition was subsequently examined, and the results are presented in Fig. 7. Fig. 7(a) shows the morphology after heat treatment under a reducing atmosphere, where a coral-like porous nanostructure was formed on the surface of the micron-sized nickel particles. In contrast, as shown in Fig. 7(b), heat treatment under ambient atmosphere preserved the original flake-like morphology of the nickel oxalate, which was oxidized and remained on the particle surface. These nanostructured coating are expected to provide enhanced reactivity due to their high specific surface area while maintaining mechanical stability.
- The overall surface modification process is summarized in Fig. 8. Nickel ions, dissolved from the precursor at the surface of pristine metallic nickel particles, undergo a precipitation reaction with oxalic acid, resulting in the formation of a nickel oxalate. Depending on the thermal treatment atmosphere, nickel oxalate is subsequently transformed into either metallic Ni or nickel oxide (NiO). This surface modification strategy significantly enhances the specific surface area through the formation of a nanostructure coating, while maintaining the thermochemical stability of the pristine nickel. The tunable phase transformation of nickel oxalate into desired nickel phases demonstrates a promising approach for the synthesis of high-performance nickel-based materials based on structural and chemical design principles.
3. Results & Discussion
- Nickel oxalate was synthesized via a solution precipitation method and subsequently converted into metallic nickel or nickel oxide depending on the heat treatment atmosphere. It was observed that the crystal structure and morphology of the nickel oxalate varied according to differences in the dielectric constant of the solvent. Following synthesis, surface modification of micron-sized nickel particles was performed using nickel oxalate, and selective phase transformation to nickel or nickel oxide under different thermal atmospheres enabled effective control over the morphology and phase of the nickel-based materials.
- The commercial nickel powder modified with nickel oxalate demonstrated the growth and morphological control of a nanoscale shell structure on the surface of the micron-sized nickel particles through heat treatment. This strategy enables the preservation of the intrinsic properties of the micron-sized substrate while simultaneously enhancing its specific surface area and reactivity by tailoring the morphology of the nanostructured coating layer. As a surface modification approach for micron-sized materials, phase and morphological control of nickel oxalate serves not only as a versatile precursor convertible into various nickel-based phases depending on synthesis and heat treatment conditions, but also offers the potential for shape regulation during precursor crystal growth. This enables a controllable foundation for tailoring the particle structure and morphology of the resulting materials.
- This study primarily focused on the synthesis of nickel oxalate as a function of solvent characteristics and heat-treatment conditions, with the discussion limited to morphological and phase evolutions. In future work, the influence of heat-treatment temperature on crystallinity and its correlation with electrochemical performance will be investigated in greater detail, particularly to evaluate the catalytic properties of the Ni/NiO phases.
4. Conclusions
-
Funding
This work was supported by the Technology Innovation Program (or Industrial Strategic Technology Development Program-Materials, Components, and Equipment Technology Development Project) (RS-2024-00469049, Development of Carbonyl Process for Producing Highly Active Nickel Powder with a Specific Surface Area of 7,000 cm2/g from Nickel Pig Iron (NPI)) funded By the Ministry of Trade Industry & Energy (MOTIE, Korea) And the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No.RS-2022-NR069413).
-
Conflict of Interest
The authors declare no conflict of interest.
-
Data Availability Statement
Data will be made available on request.
-
Author Information and Contribution
Eunbi Park: MSc students; Analyzed the data and wrote the draft of the manuscript.
Jongwon Bae: MSc students; Assisted with the experiments.
Sera Kang: MSc students; Assisted with the experiments.
Minsu Kang: MSc students; Assisted with the experiments.
Suseong Lee: MSc students; Assisted with the experiments.
Kun-Jae Lee: Professor; Provided overall supervision; Conceived and designed the experiments; Reviewing and editing the final manuscript.
-
Acknowledgments
None.
Article information
- 1. N. Mahmood, Y. Yao, J. W. Zhang, L. Pan, X. Zhang and J. J. Zou: Adv. Sci., 5 (2018) 1700464.Article
- 2. W. U. Arifeen, M. K. Aslam, M. Ahmad, S. Reshail, P. Rosaiah, Y. S. Usmani, I. Hussain and T. J. Ko: J. Electroanal. Chem., 978 (2025) 118882.Article
- 3. H. Shin, J. Bae, M. Kang and K.-J. Lee: J. Powder Mater., 30 (2023) 502.Article
- 4. X.-M. Liu and S.-Y. Fu: J. Cryst. Growth, 306 (2007) 428.Article
- 5. C.-W. Won, J.-H. Bae, J.-H. Lee and B.-B. Kim: J. Korean Powder Metall. Inst., 11 (2004) 217.Article
- 6. O. A. Logutenko, A. I. Titkov, A. M. Vorob’yov, D. A. Balaev, K. A. Shaikhutdinov, S. V. Semenov, Y. M. Yukhin and N. Z. Lyakhov: Eur. Polym. J, 99 (2018) 102.Article
- 7. J. Wang, L. Wei, L. Zhang, Y. Zhang and C. Jiang: CrystEngComm, 14 (2012) 1629.Article
- 8. K.-H. Ryu, H.-S. So, J.-S. Yoon, I.-H. Kim and K.-J. Lee: J. Korean Powder Metall. Inst., 26 (2019) 201.Article
- 9. S. Chenakin and N. Kruse: J. Phys. Chem. C, 123 (2019) 30926.Article
- 10. D.-W. Kim, N.-K. Ahn, H.-W. Shim, K.-S. Park and H.-L. Choi: J. Korean Powder Metall. Inst., 25 (2018) 213.
- 11. H. Ma, Z. Liu and Z. Liu: RSC Adv., 12 (2022) 15251.Article
- 12. H.-L. Yue, H.-Y. Zeng, J.-F. Peng, W. Yan, K. Zhang, C.-W. Luo and Z.-F. Tian: J. Colloid Interface Sci., 678 (2025) 221.Article
- 13. Z. Liu, W. Yuan, H. Yang, Z. Kang, M. Ma, P. W. Menezes and Z. Chen: Adv. Funct. Mater., 35 (2025) 2412198.Article
- 14. Y. B. Kim, S. Kim, Y. Hong, J. Lee, H. Sun and W. Jung: Carbon Energy, 7 (2025) e696.Article
- 15. Z. Xu, Q. Song, Y. Fang, H. Xie, Y. Du, X. Tang, Z. Qi, J. Yang, Y. Song and L. Guo: ACS Sustainable Chem. Eng., 10 (2022) 10991.
- 16. R. Yi, H. Wang and X. Wang: Mater. Sci. Eng. B, 178 (2013) 1.
- 17. G. Kim, Y. Son, Y. Jeong, M. Kim and G. Lee: J. Electroanal. Chem., 977 (2025) 118826.Article
- 18. S. V. Yukhina, Y. M. Yukhin and N. Z. Lyakhov: Eur. Polym. J., 99 (2018) 102.Article
- 19. D.-P. Wang, D.-B. Sun, H.-Y. Yu and H.-M. Meng: J. Cryst. Growth, 310 (2008) 1195.Article
- 20. S. Vaidya, P. Rastogi, S. Agarwal, S. K. Gupta, T. Ahmad, A. M. Antonelli, K. V. Ramanujachary, S. E. Lofland and A. K. Ganguli: J. Phys. Chem. C, 112 (2008) 12610.Article
- 21. H. Kwon, M. K. Kabiraz, J. Park, S.-I. Choi and K. Lee: Nano Lett., 18 (2018) 2930.Article
- 22. J. Zhu, L. Hu, P. Zhao, L. Y. S. Lee and K.-Y. Wong: Chem. Rev., 120 (2020) 851.Article
- 23. F. Sun, T. Chen, Q. Li and H. Pang: J. Colloid Interface Sci., 627 (2022) 483.Article
- 24. L. B. McCusker, R. B. Von Dreele, D. E. Cox, D. Louër and P. Scardi: J. Appl. Cryst., 32 (1999) 36.Article
- 25. I. Jung, J. Choi and Y. Tak: J. Mater. Chem., 20 (2010) 6164.Article
- 26. F. Behnoudnia and H. Dehghani: Dalton Trans., 43 (2014) 3471.Article
References
Figure & Data
References
Citations

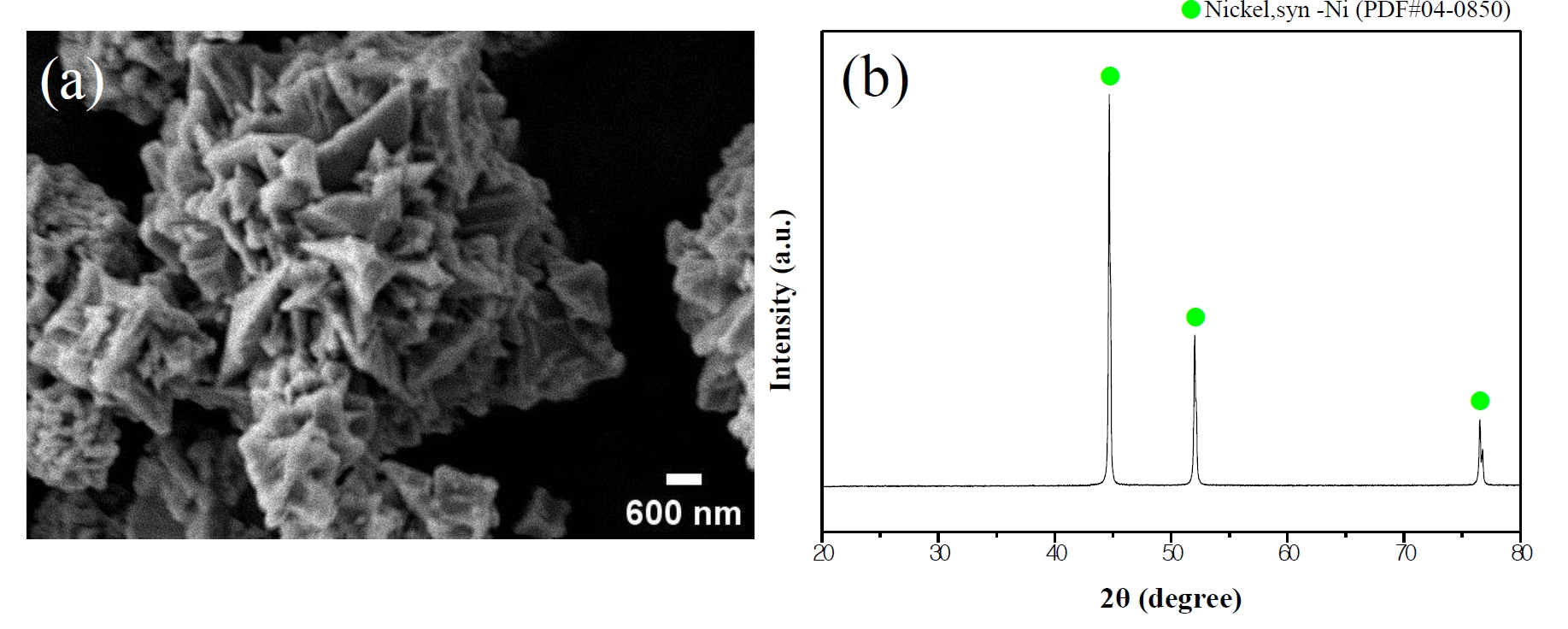
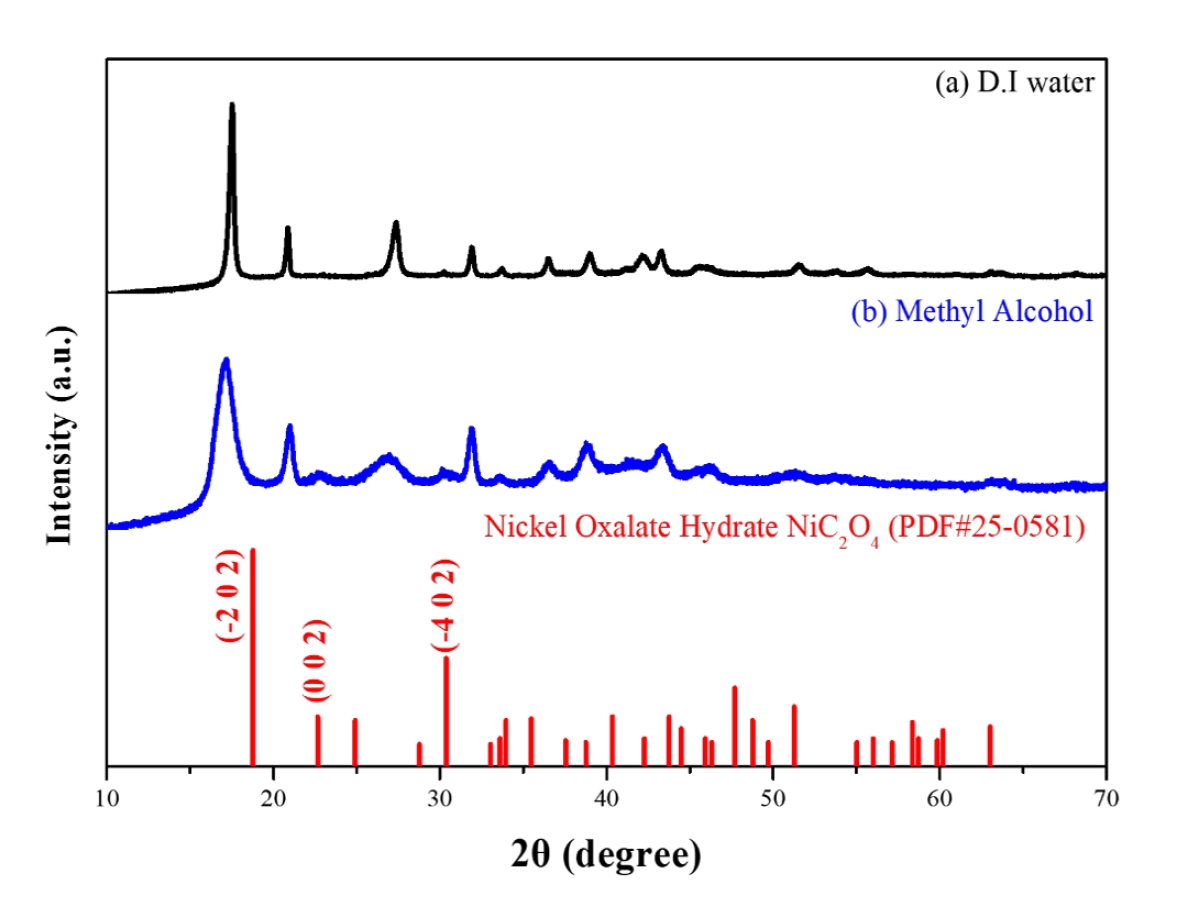
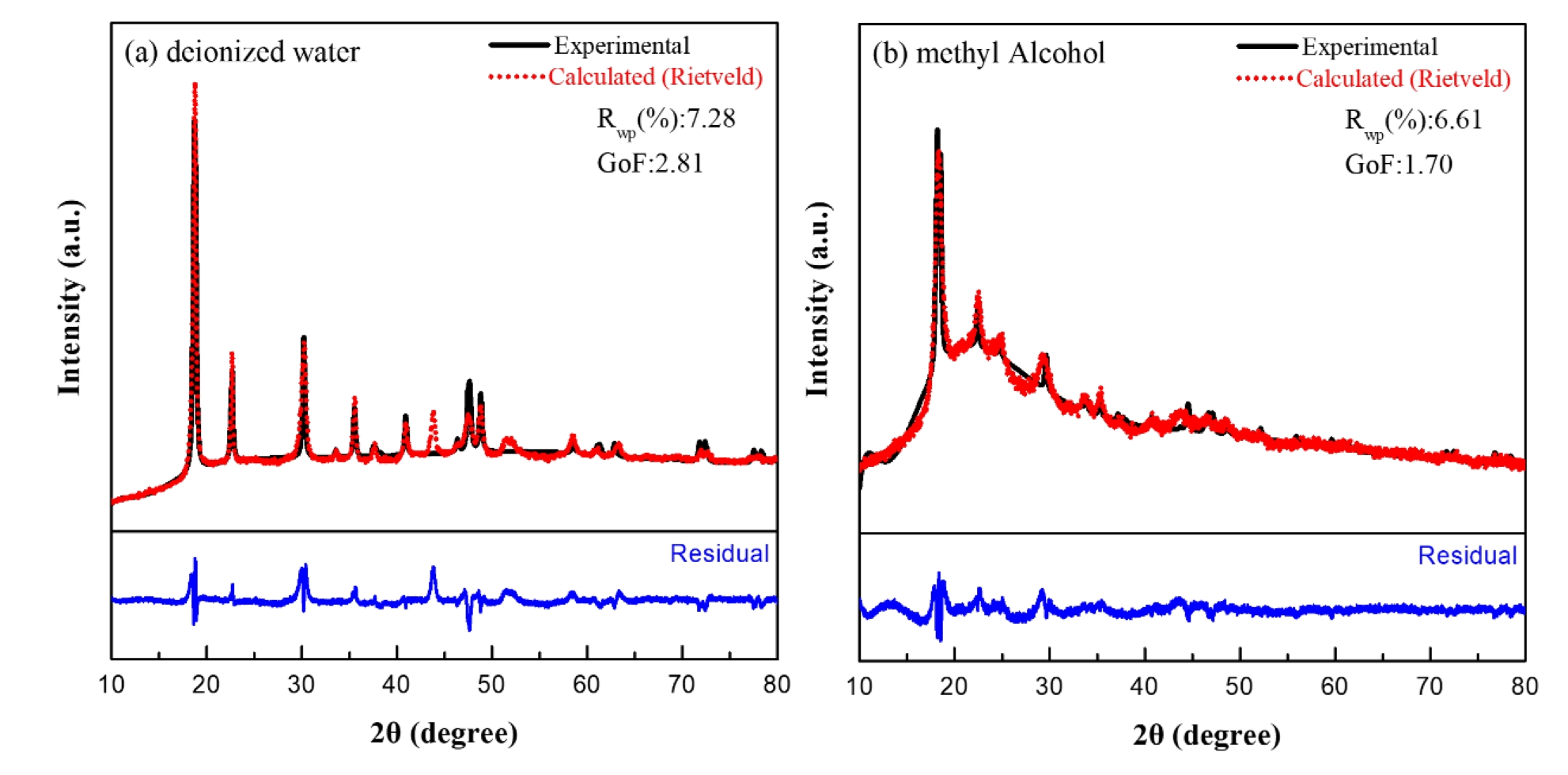
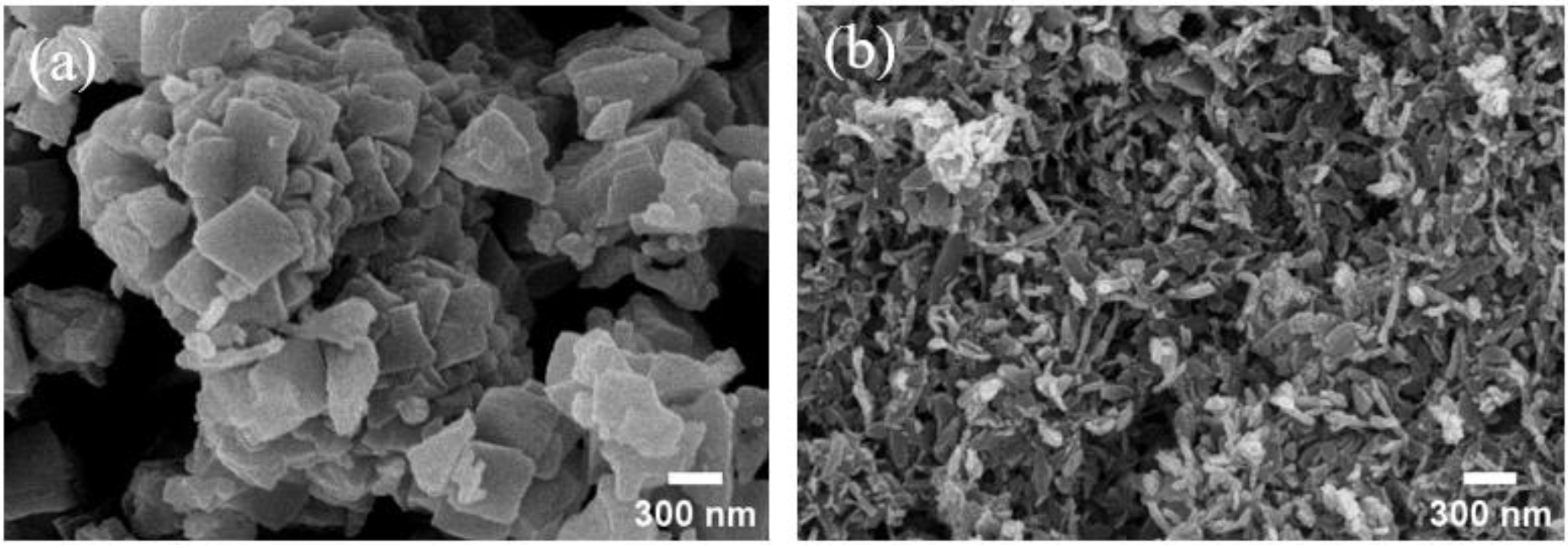
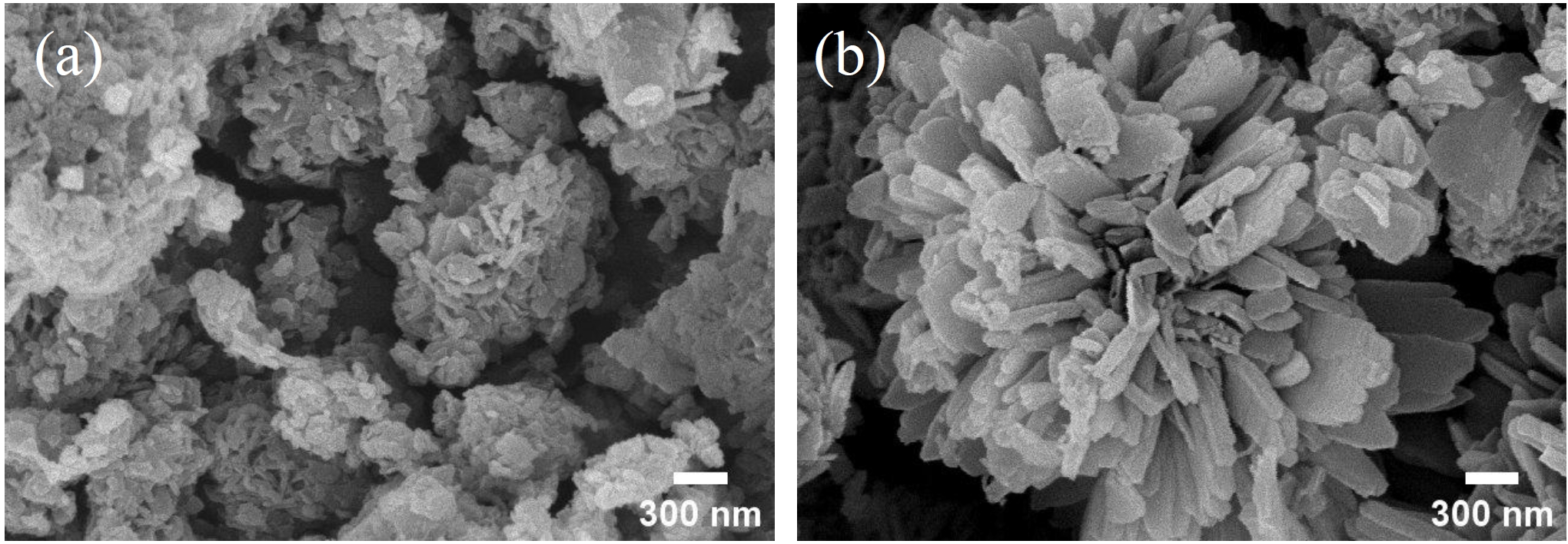
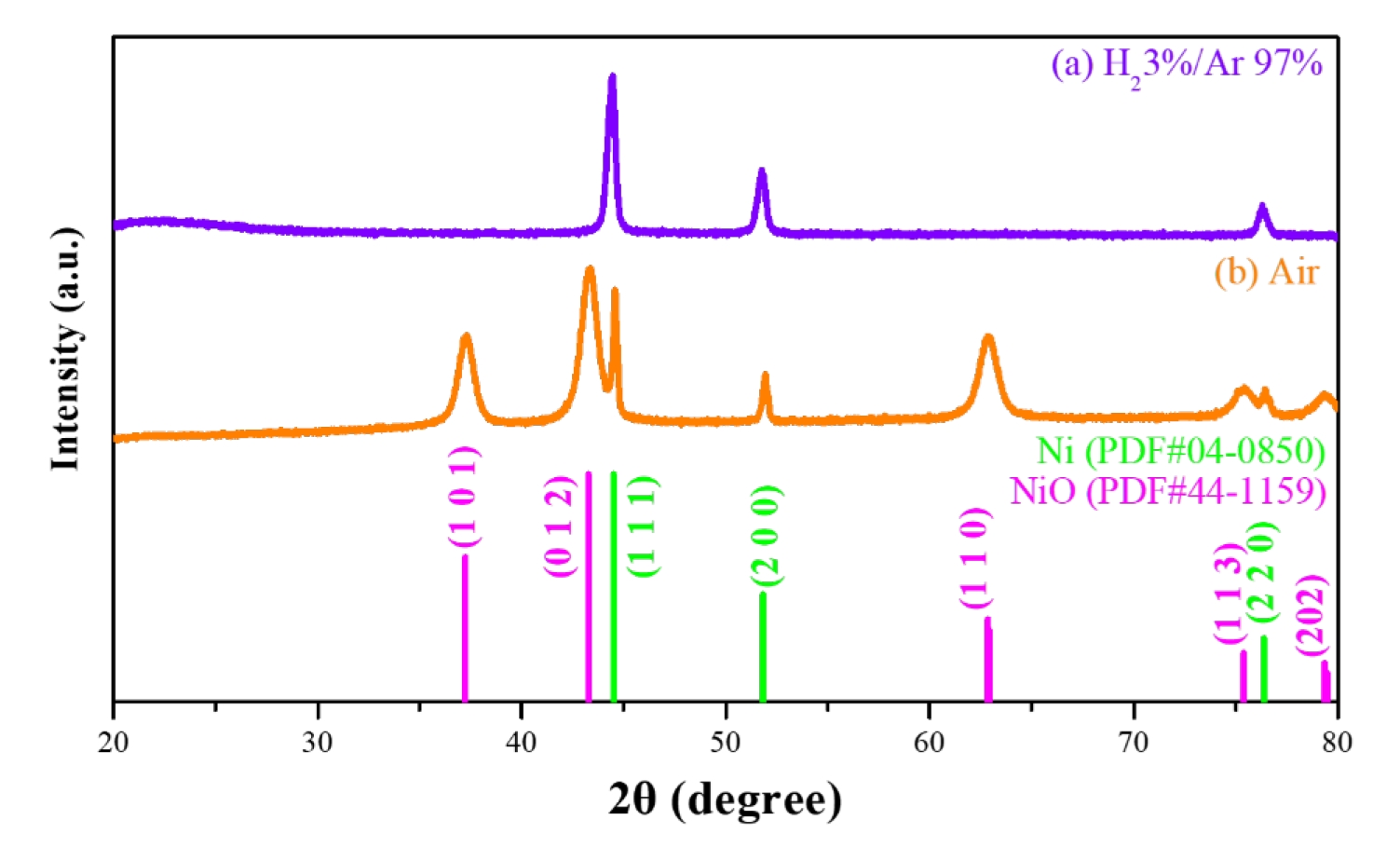
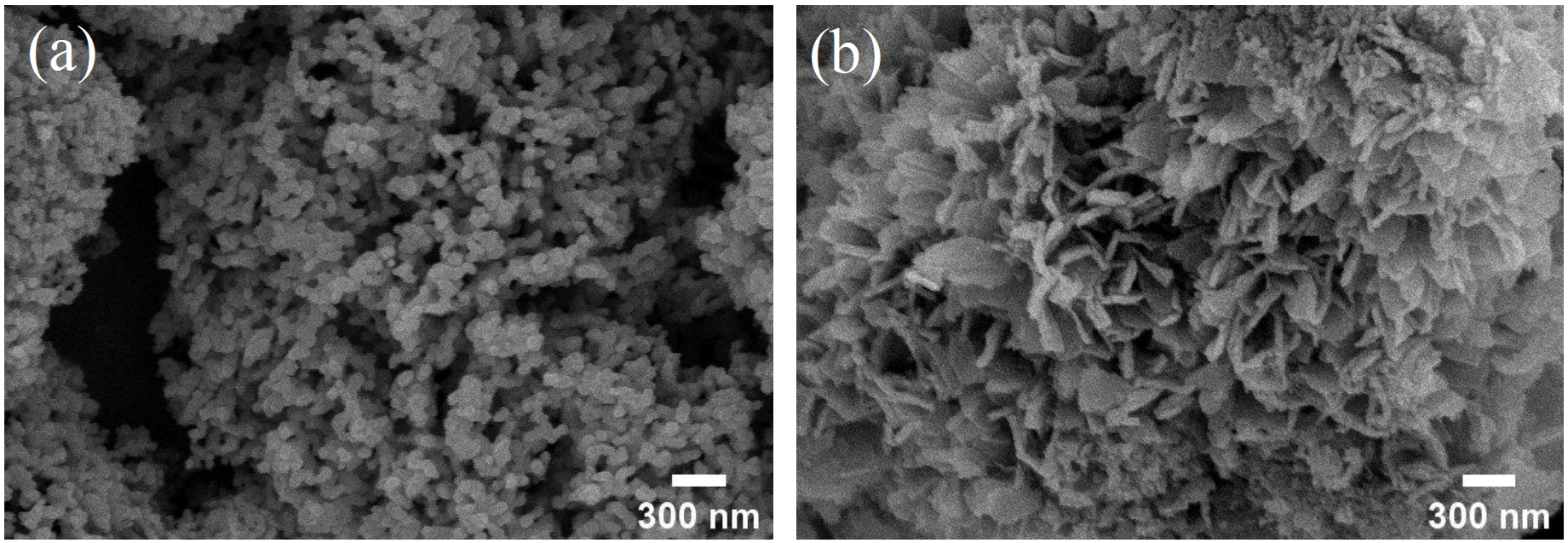
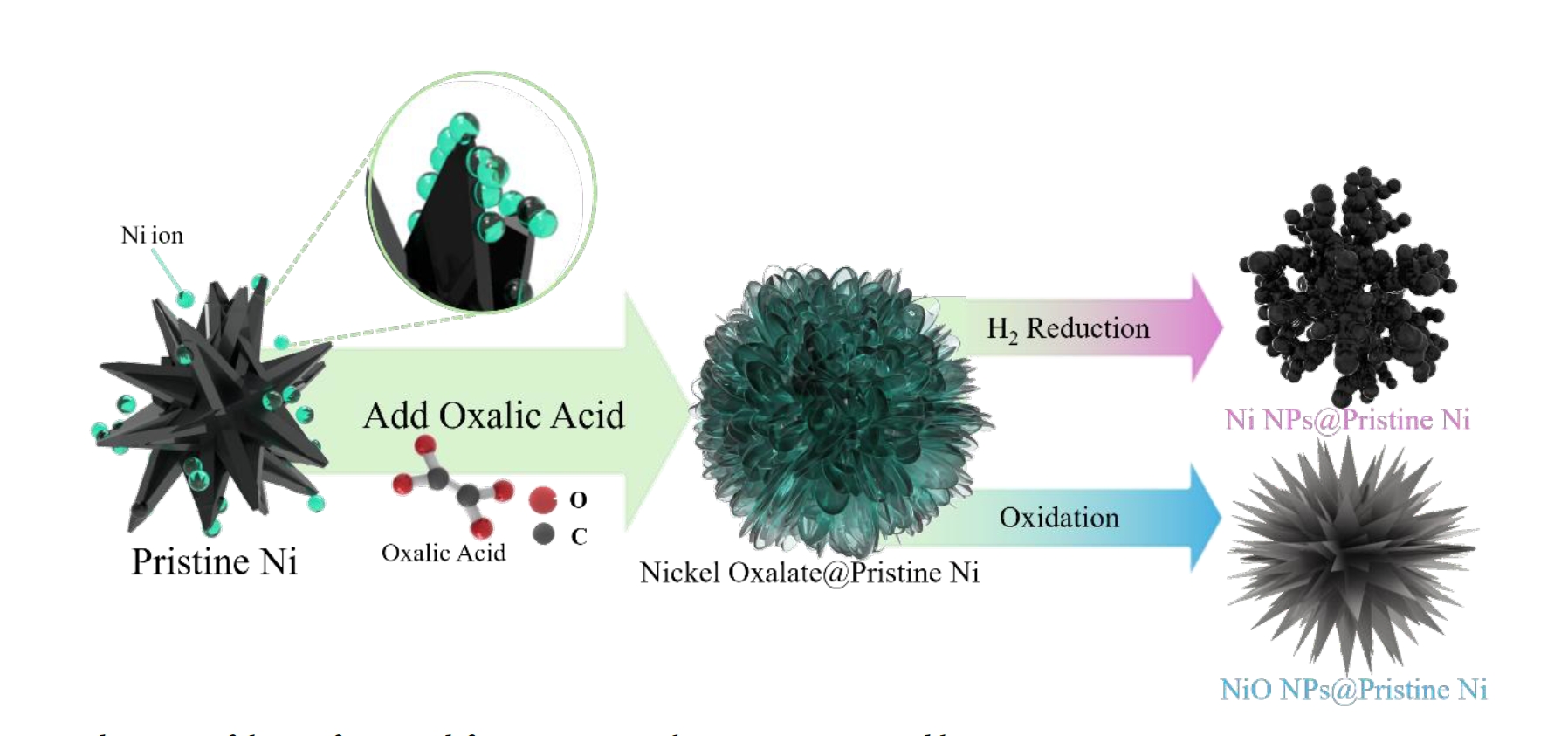
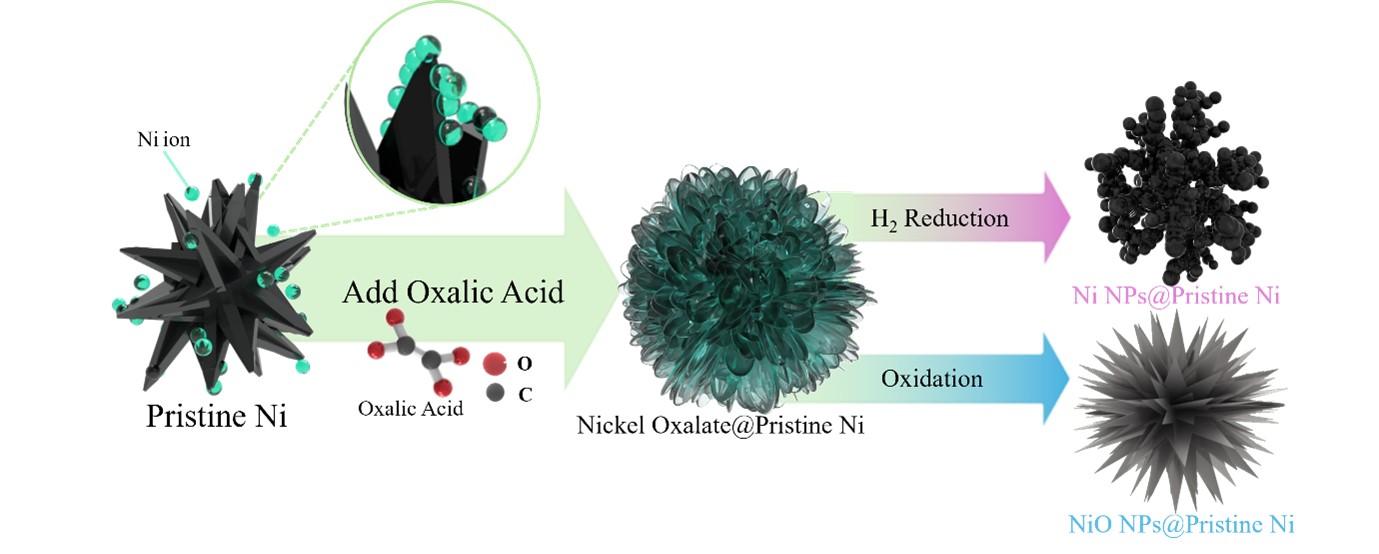
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
Fig. 7.
Fig. 8.
Graphical abstract
TOP
 KPMI
KPMI

 ePub Link
ePub Link Cite this Article
Cite this Article









