Search
- Page Path
- HOME > Search
- [Korean]
- Preparation of Spherical Cobalt Fine Powders by New Liquid Reduction Method
- Dae Weon Kim, Ji-Hoon Kim, Yo-Han Choi, Hee Lack Choi, Jin-Ho Yoon
- J Korean Powder Metall Inst. 2015;22(4):260-265. Published online August 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.4.260
- 714 View
- 5 Download
-
 Abstract
Abstract
 PDF
PDF Spherical fine cobalt powders were fabricated by new liquid reduction method. Commercial cobalt sufate heptahydrate was used as raw material. Also ethylene glycol was used as solvent and hydrazine-sodium hypophosphite mixture was used as reduction agent for the new liquid reduction method. A plate shaped cobalt powders with an approximately 300 nm were prepared by a traditional wet ruduction method using distilled water as solvent and hydrazine. Spherical fine cobalt powders with an average size of 1-3 μm were synthesized by a new liquid reduction method in 0.3M cobalt sulfate and 1.5M hydrazine-0.6M sodium hypophosphite mixture at 333K.
- [Korean]
- Preparation of Cathode Materials for Lithium Rechargeable Batteries using Transition Metals Recycled from Li(Ni1-x-yCoxMny)O2 Secondary Battery Scraps
- Jae-won Lee, Dae Weon Kim, Seong Tae Jang
- J Korean Powder Metall Inst. 2014;21(2):131-136. Published online April 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.2.131
- 581 View
- 3 Download
-
 Abstract
Abstract
 PDF
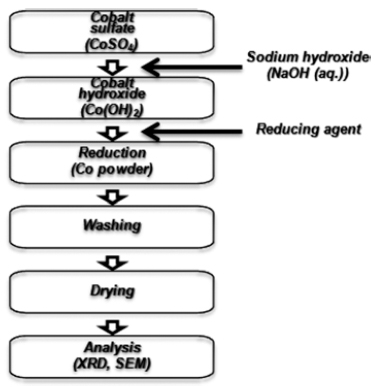
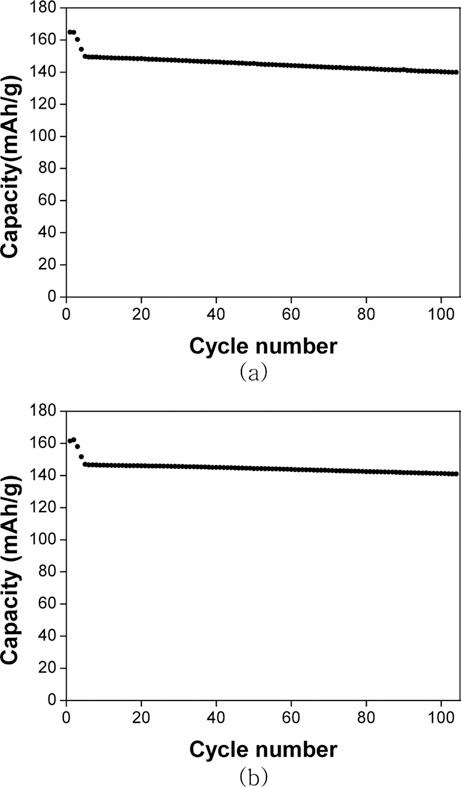
PDF Cathode materials and their precursors are prepared with transition metal solutions recycled from the the waste lithium-ion batteries containing NCM (nickel-cobalt-manganese) cathodes by a H2 and C-reduction process. The recycled transition metal sulfate solutions are used in a co-precipitation process in a CSTR reactor to obtain the transition metal hydroxide. The NCM cathode materials (Ni:Mn:Co=5:3:2) are prepared from the transition metal hydroxide by calcining with lithium carbonate. X-ray diffraction and scanning electron microscopy analyses show that the cathode material has a layered structure and particle size of about 10 μm. The cathode materials also exhibited a capacity of about 160 mAh/g with a retention rate of 93~96% after 100 cycles.
TOP
 KPMI
KPMI

 First
First Prev
Prev


