Search
- Page Path
- HOME > Search
- [Korean]
- Effect of Surfactant on the Dispersion Stability of Slurry for Semiconductor Silicon CMP
- Hye Won Yun, Doyeon Kim, Do Hyung Han, Dong Wan Kim, Woo-Byoung Kim
- J Korean Powder Metall Inst. 2018;25(5):395-401. Published online October 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.5.395
- 2,118 View
- 46 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
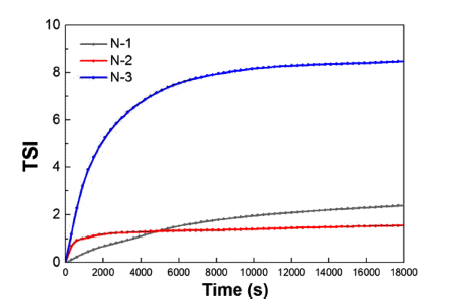
PDF The improvement of dispersion stability for the primary polishing slurry in a CMP process is achieved to prevent defects produced by agglomeration of the slurry. The dispersion properties are analyzed according to the physical characteristics of each silica sol sample. Further, the difference in the dispersion stability is confirmed as the surfactant content. The dispersibility results measured by Zeta potential suggest that the dispersion properties depend on the content and size of the abrasive in the primary polishing slurry. Moreover, the optimum ratio for high dispersion stability is confirmed as the addition content of the surfactant. Based on the aforementioned results, the long-term stability of each slurry is analyzed. Turbiscan analysis demonstrates that the agglomeration occurs depending on the increasing amount of surfactant. As a result, we demonstrate that the increased particle size and the decreased content of silica improve the dispersion stability and long-term stability.
-
Citations
Citations to this article as recorded by- Surface Defect Properties of Prime, Test-Grade Silicon Wafers
Seung-Hwan Oh, Hyeonmin Yim, Donghee Lee, Dong Hyeok Seo, Won Jin Kim, Ryun Na Kim, Woo-Byoung Kim
Korean Journal of Materials Research.2022; 32(9): 396. CrossRef
- Surface Defect Properties of Prime, Test-Grade Silicon Wafers
- [Korean]
- Study on Surface-defect Passivation of InP System Quantum Dots by Photochemical Method
- Doyeon Kim, Hyun-Su Park, Hye Mi Cho, Bum-Sung Kim, Woo-Byoung Kim
- J Korean Powder Metall Inst. 2017;24(6):489-493. Published online December 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.6.489
- 1,595 View
- 12 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
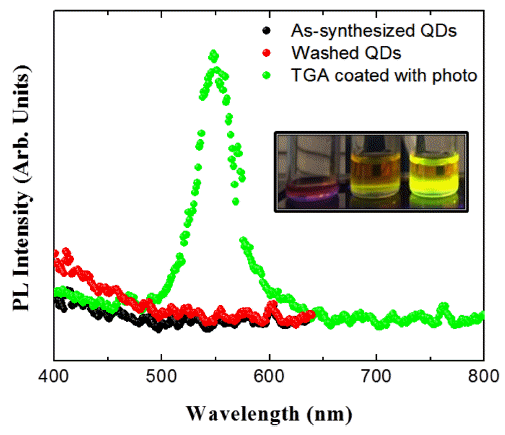
PDF In this study, the surface passivation process for InP-based quantum dots (QDs) is investigated. Surface coating is performed with poly(methylmethacrylate) (PMMA) and thioglycolic acid. The quantum yield (QY) of a PMMA-coated sample slightly increases by approximately 1.3% relative to that of the as-synthesized InP/ZnS QDs. The QYs of the uncoated and PMMA-coated samples drastically decrease after 16 days because of the high defect state density of the InP-based QDs. PMMA does not have a significant effect on the defect passivation. Thioglycolic acid is investigated in this study for the effective surface passivation of InP-based QDs. Surface passivation with thioglycolic acid is more effective than that with the PMMA coating, and the QY increases from 1.7% to 11.3%. ZnS formed on the surface of the InP QDs and S in thioglycolic acid show strong bonding property. Additionally, the QY is further increased up to 21.0% by the photochemical reaction. Electron–hole pairs are formed by light irradiation and lead to strong bonding between the inorganic and thioglycolic acid sulfur. The surface of the InP core QDs, which does not emit light, is passivated by the irradiated light and emits green light after the photochemical reaction.
-
Citations
Citations to this article as recorded by- Poly(methylmethacrylate) coating on quantum dot surfaces via photo-chemical reaction for defect passivation
Doyeon Kim, So-Yeong Joo, Chan Gi Lee, Bum-Sung Kim, Woo-Byoung Kim
Journal of Photochemistry and Photobiology A: Chemistry.2019; 376: 206. CrossRef
- Poly(methylmethacrylate) coating on quantum dot surfaces via photo-chemical reaction for defect passivation
TOP
 KPMI
KPMI


 First
First Prev
Prev


