Search
- Page Path
- HOME > Search
- [Korean]
- Photocatalytic and Adsorption Properties of WO3 Nanorods Prepared by Hydrothermal Synthesis
- Su-Yeol Yu, Chunghee Nam
- J Korean Powder Metall Inst. 2017;24(6):483-488. Published online December 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.6.483
- 1,110 View
- 4 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
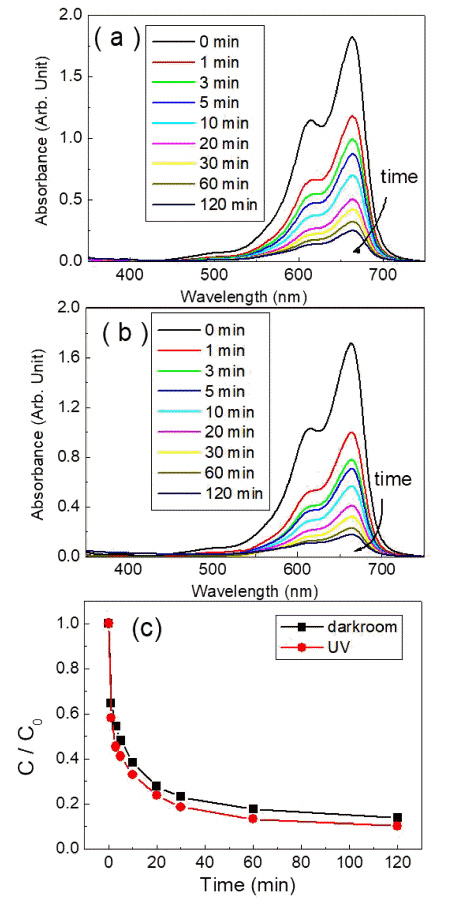
PDF Transition-metal oxide semiconductors have various band gaps. Therefore, many studies have been conducted in various application fields. Among these, methods for the adsorption of organic dyes and utilization of photocatalytic properties have been developed using various metal oxides. In this study, the adsorption and photocatalytic effects of WO3 nanomaterials prepared by hydrothermal synthesis are investigated, with citric acid added in the hydrothermal process as a structure-directing agent. The nanostructures of WO3 are studied using transmission electron microscopy and scanning electron microscopy images. The crystal structure is investigated using X-ray diffraction patterns, and the changes in the dye concentrations adsorbed on WO3 nanorods are measured with a UV-visible absorption spectrophotometer based on Beer-Lambert’s law. The methylene blue (MB) dye solution is subjected to acid or base conditions to monitor the change in the maximum adsorption amount in relation to the pH. The maximum adsorption capacity is observed at pH 3. In addition to the dye adsorption, UV irradiation is carried out to investigate the decomposition of the MB dye as a result of photocatalytic effects. Significant photocatalytic properties are observed and compared with the adsorption effects for dye removal.
-
Citations
Citations to this article as recorded by- Photocatalytic Properties of WO3 Thin Films Prepared by Electrodeposition Method
Kwang-Mo Kang, Ji-Hye Jeong, Ga-In Lee, Jae-Min Im, Hyun-Jeong Cheon, Deok-Hyeon Kim, Yoon-Chae Nah
Journal of Korean Powder Metallurgy Institute.2019; 26(1): 40. CrossRef - Photocatalysis of TiO<sub>2</sub>/WO<sub>3</sub> Composites Synthesized by Ball Milling
Su-Yeol Yu, Chunghee Nam
Journal of Korean Powder Metallurgy Institute.2018; 25(4): 316. CrossRef
- Photocatalytic Properties of WO3 Thin Films Prepared by Electrodeposition Method
TOP
 KPMI
KPMI


 First
First Prev
Prev


