Search
- Page Path
- HOME > Search
- [Korean]
- A Study on the Preparation and Growth Mechanism of Titanium Dioxide using Organic-Inorganic Hybrid Titanium Complex
- Yubin Kang, Jin-Ju Choi, Nam Hun Kwon, Dae-Guen Kim, Kun-Jae Lee
- J Korean Powder Metall Inst. 2019;26(6):487-492. Published online December 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.6.487
- 1,149 View
- 15 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
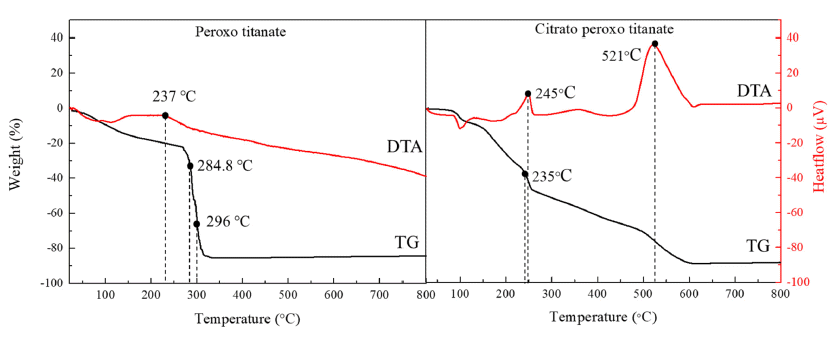
PDF Titanium dioxide (TiO2) is a typical inorganic material that has an excellent photocatalytic property and a high refractive index. It is used in water/air purifiers, solar cells, white pigments, refractory materials, semiconductors, etc.; its demand is continuously increasing. In this study, anatase and rutile phase titanium dioxide is prepared using hydroxyl and carboxyl; the titanium complex and its mechanism are investigated. As a result of analyzing the phase transition characteristics by a heat treatment temperature using a titanium complex having a hydroxyl group and a carboxyl group, it is confirmed that the material properties were different from each other and that the anatase and rutile phase contents can be controlled. The titanium complexes prepared in this study show different characteristics from the titania-formation temperatures of the known anatase and rutile phases. It is inferred that this is due to the change of electrostatic adsorption behavior due to the complexing function of the oxygen sharing point, which crystals of the TiO6 structure share.
-
Citations
Citations to this article as recorded by- Thermal Stability and Weight Reduction of Al0.75V2.82CrZr Refractory High Entropy Alloy Prepared Via Mechanical Alloying
Minsu Kim, Hansung Lee, Byungmin Ahn
journal of Korean Powder Metallurgy Institute.2023; 30(6): 478. CrossRef
- Thermal Stability and Weight Reduction of Al0.75V2.82CrZr Refractory High Entropy Alloy Prepared Via Mechanical Alloying
- [Korean]
- Recent Development in Fabrication and Control of Layered-Double Hydroxide Nanostructures
- Chan-Woo Jeon, Il-Kyu Park
- J Korean Powder Metall Inst. 2018;25(6):514-522. Published online December 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.6.514
- 1,844 View
- 25 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
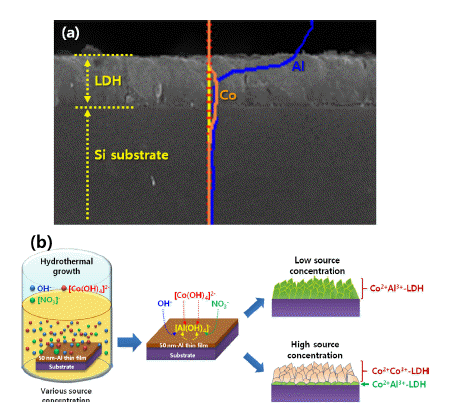
PDF Layered-double hydroxide (LDH)-based nanostructures offer the two-fold advantage of being active catalysts with incredibly large specific surface areas. As such, they have been studied extensively over the last decade and applied in roles as diverse as light source, catalyst, energy storage mechanism, absorber, and anion exchanger. They exhibit a unique lamellar structure consisting of a wide variety of combinations of metal cations and various anions, which determine their physical and chemical performances, and make them a popular research topic. Many reviewed papers deal with these unique properties, synthetic methods, and applications. Most of them, however, are focused on the form-factor of nanopowder, as well as on the control of morphologies via one-step synthetic methods. LDH nanostructures need to be easy to control and fabricate on rigid substrates such as metals, semiconductors, oxides, and insulators, to facilitate more viable applications of these nanostructures to various solid-state devices. In this review, we explore ways to grow and control the various LDH nanostructures on rigid substrates.
-
Citations
Citations to this article as recorded by- Review of Domestic Research Trends on Layered Double Hydroxide (LDH) Materials: Based on Research Articles in Korean Citation Index (KCI)
Seon Yong Lee, YoungJae Kim, Young Jae Lee
Economic and Environmental Geology.2023; 56(1): 23. CrossRef
- Review of Domestic Research Trends on Layered Double Hydroxide (LDH) Materials: Based on Research Articles in Korean Citation Index (KCI)
- [Korean]
- The Preparation and Growth Mechanism of the Recovered Bi2Te3 Particles with Respect to Surfactants
- Hyeongsub So, Eunpil Song, Yong-Ho Choa, Kun-Jae Lee
- J Korean Powder Metall Inst. 2017;24(2):141-146. Published online April 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.2.141

- 1,187 View
- 9 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Bi2Te3 powders are recovered by wet chemical reduction for waste n-type thermoelectric chips, and the recovered particles with different morphologies are prepared using various surfactants such as cetyltrimethylammonium bromide (CTAB), sodium dodecylbenzenesulfonate (SDBS), and ethylenediaminetetraacetic acid (EDTA). When citric acid is added as the surfactant, the shape of the aggregated particles shows no distinctive features. On the other hand, rod-shaped particles are formed in the sample with CTAB, and sheet-like particles are synthesized with the addition of SDBS. Further, particles with a tripod shape are observed when EDTA is added as the surfactant. The growth mechanism of the particle shapes depending on the surfactant is investigated, with a focus on the nucleation and growth phenomena. These results help to elucidate the intrinsic formation mechanism of the rod, plate, and tripod structures of the Bi2Te3 recovered by the wet reduction process.
-
Citations
Citations to this article as recorded by- Recovery of Barium, Nickel, and Titanium Powders from Waste MLCC
Haein Shin, Kun-Jae Lee
Journal of Powder Materials.2024; 31(5): 374. CrossRef
- Recovery of Barium, Nickel, and Titanium Powders from Waste MLCC
- [Korean]
- Growth mechanism of InP and InP/ZnS synthesis using colloidal synthesis
- Han wook Seo, Da-woon Jeong, Bin Lee, Seoung kyun Hyun, Bum Sung Kim
- J Korean Powder Metall Inst. 2017;24(1):6-10. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.6
- 1,447 View
- 5 Download
-
 Abstract
Abstract
 PDF
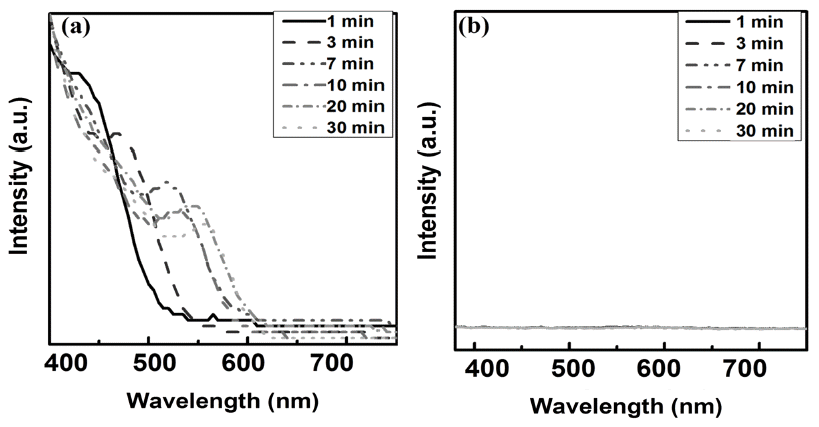
PDF This study investigates the main growth mechanism of InP during InP/ZnS reaction of quantum dots (QDs). The size of the InP core, considering a synthesis time of 1-30 min, increased from the initial 2.56 nm to 3.97 nm. As a result of applying the proposed particle growth model, the migration mechanism, with time index 7, was found to be the main reaction. In addition, after the removal of unreacted In and P precursors from bath, further InP growth (of up to 4.19 nm (5%)), was observed when ZnS was added. The full width at half maximum (FWHM) of the synthesized InP/ZnS quantum dots was found to be relatively uniform, measuring about 59 nm. However, kinetic growth mechanism provides limited information for InP / ZnS core shell QDs, because the surface state of InP changes with reaction time. Further study is necessary, in order to clearly determine the kinetic growth mechanism of InP / ZnS core shell QDs.
TOP
 KPMI
KPMI


 First
First Prev
Prev


