Search
- Page Path
- HOME > Search
- [Korean]
- Study on preparation and photocatalytic properties of F-containing TiO2 nanopowders using wet-process from Ammonium Hexafluorotitanate
- Duk-Hee Lee, Jae-Ryang Park, Chan-Gi Lee, Hyeon-Mo Kim, Kyung-Soo Park
- J Korean Powder Metall Inst. 2018;25(3):226-331. Published online June 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.3.226

- 510 View
- 2 Download
-
 Abstract
Abstract
 PDF
PDF F-containing TiO2 nanopowders are synthesized using simple wet processes (precipitation-based and hydrothermal) from ammonium hexafluorotitanate (AHFT, (NH4)2TiF6) as a precursor to apply as a photocatalyst for the degradation of rhodamine B (RhB). The surface properties of the prepared samples are evaluated using X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), field-emission scanning electron microscopy (FESEM), and transmission electron microscopy (TEM). The results confirm that the synthesized anatase TiO2 has sphere-like shapes, with numerous small nanoparticles containing fluorine on the surface. The photocatalytic activity of F-containing TiO2 compared with F-free TiO2 is characterized by measuring the degradation of RhB using a xenon lamp. The photocatalytic degradation of F-containing TiO2 exhibits improved photocatalytic activity, based on the positive effects of adsorbed F ions on the surface.
- [Korean]
- Study on thermal behavior of Ammonium Hexafluofide Titanate for Synthesis of TiO2 Powders
- Duk-Hee Lee, Jae-Ryang Park, Chan-Gi Lee, Kyung-Soo Park, Hyeon-Mo Kim
- J Korean Powder Metall Inst. 2016;23(5):353-357. Published online October 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.5.353
- 577 View
- 1 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
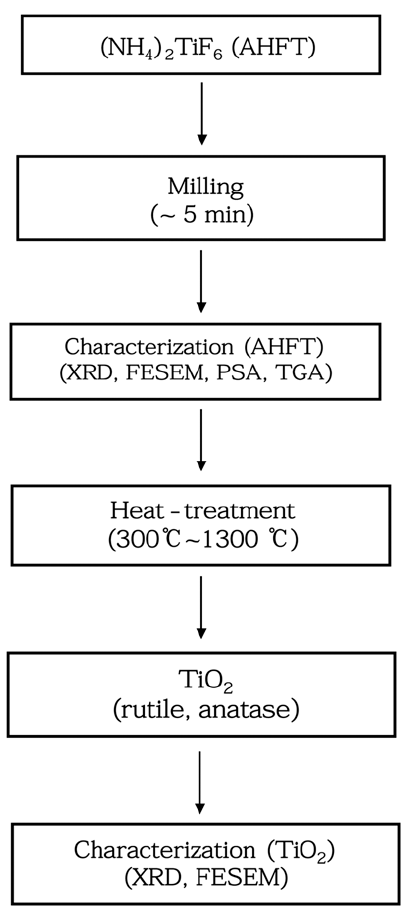
PDF In this study, TiO2 powders are synthesized from ammonium hexafluoride titanate (AHFT, (NH4)2TiF6) as a precursor by heat treatment. First, we evaluate the physical properties of AHFT using X-ray diffraction (XRD), particle size analysis (PSA), thermogravimetric analysis (TGA), and field-emission scanning electron microscopy (FESEM). Then, to prepare the TiO2 powders, is heat-treated at 300-1300°C for 1 h. The ratio of anatase to rutile phase in TiO2 is estimated by XRD. The anatase phase forms at 500°C and phase transformation to the rutile phase occurs at 1200°C. Increase in the particle size is observed upon increasing the reaction temperature, and the phase ratio of the rutile phase is determined from a comparison with the calculated XRD data. Thus, we show that anatase and rutile TiO2 powders could be synthesized using AHFT as a raw material, and the obtained data are utilized for developing a new process for producing high-quality TiO2 powder.
-
Citations
Citations to this article as recorded by- Photocatalytic activity of rutile TiO2 powders coupled with anatase TiO2 nanoparticles using surfactant
Jong Min Byun, Chun Woong Park, Young In Kim, Young Do Kim
journal of Korean Powder Metallurgy Institute.2018; 25(3): 257. CrossRef
- Photocatalytic activity of rutile TiO2 powders coupled with anatase TiO2 nanoparticles using surfactant
TOP
 KPMI
KPMI


 First
First Prev
Prev


