Search
- Page Path
- HOME > Search
- [English]
- Influences of the Eu Concentration and the Milling Time on Photoluminescence Properties of Y2O3-H3BO3:Eu3+ Powders Prepared by Mechanical Alloying
- Hyun-Sic Gong, Hyun-Goo Kim
- J Korean Powder Metall Inst. 2016;23(2):108-111. Published online April 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.2.108
- 616 View
- 3 Download
-
 Abstract
Abstract
 PDF
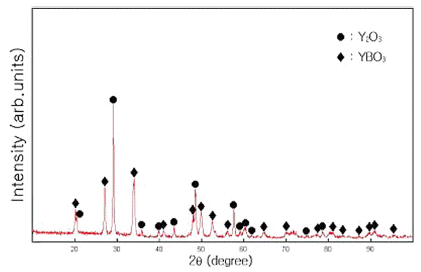
PDF Y2O3–H3BO3:Eu3+ powders are synthesized using a mechanical alloying method, and their photoluminescence (PL) properties are investigated through luminescence spectrophotometry. For samples milled for 300 min, some Y2O3 peaks ([222], [440], and [622]) and amorphous formations are observed. The 300-min-milled mixture annealed at 800°C for 1 h with Eu = 8 mol% has the strongest PL intensity at every temperature increase of 100°C (increasing from 700 to 1200°C in 100°C increments). PL peaks of the powder mixture, as excited by a xenon discharge lamp (20 kW) at 240 nm, are detected at approximately 592 nm (orange light, 5Do → 7F1), 613 nm, 628 nm (red light, 5Do → 7F1), and 650 nm. The PL intensity of powder mixtures milled for 120 min is generally lower than that of powder mixtures milled for 300 min under the same conditions. PL peaks due to YBO3 and Y2O3 are observed for 300-min-milled Y2O3–H3BO3 with Eu = 8 mol% after annealing at 800°C for 1 h.
- [Korean]
- Formation and Thermal Properties of Amorphous Ti40Cu40Ni10Al10 Alloy by Mechanical Alloying
- Hyun-Goo Kim
- J Korean Powder Metall Inst. 2009;16(5):363-369.
- DOI: https://doi.org/10.4150/KPMI.2009.16.5.363

- 494 View
- 0 Download
-
 Abstract
Abstract
 PDF
PDF - The amorphization process and the thermal properties of amorphous Ti_40Cu_40Ni_10Al_10 powder during milling by mechanical alloying were examined by X-ray diffractometry (XRD), differential scanning calorimetry (DSC), and transmission electron microscopy (TEM). The chemical composition of the samples was examined by an energy dispersive X-ray spectrometry (EDX) facility attached to the scanning electron microscope (SEM). The as-milled powders showed a broad peak (2theta = 42.4°) with crystalline size of about 5.0 nm in the XRD patterns. The entire milling process could be divided into three different stages: agglomeration (0 < t_m ≤ 3 h), disintegration (3 h < t_m ≤ 20 h), and homogenization (20 h < t_m ≤ 40 h) (t_m: milling time). In the DSC experiment, the peak temperature T_p and crystallization temperature T_x were 466.9°C and 444.3°C, respectively, and the values of T_p, and T_x increased with a heating rate (HR). The activation energies of crystallization for the as-milled powder was 291.5 kJ/mol for T_p.
- [Korean]
- Crystallization and Magnetic Properties of Non-Equilibrium Al(Fe-Cu) Alloy Powders Produced by Rod Milling and Chemical Leaching
- Hyun-Goo Kim
- J Korean Powder Metall Inst. 2004;11(6):486-492.
- DOI: https://doi.org/10.4150/KPMI.2004.11.6.486

- 478 View
- 0 Download
-
 Abstract
Abstract
 PDF
PDF - We report the crystallization and magnetic properties of non-equilibrium Al_0.6(Fe_xCu_1-x)_0.4(x=0.25, 0.50, 0.75) alloy powders produced by rod-milling as well as by new chemical leaching. X-ray diffractometry, transmission electron microscopy, differential scanning calorimetry and vibrating sample magnetometry were used to characterize the as-milled and leached specimens. After 400 h or 500 h milling, only the broad peaks of nano bcc crystalline phases were detected in the XRD patterns. The crystallite size, the peak and the crystallization temperatures increased with increasing Fe. After being annealed at 600circC for 1 h for as-milled alloy powders, the peaks of bcc AlCu_4;and;Al_13Cu_4Fe_3;for;x=0.25,;bcc;AlCu_4;and;Al_5Fe_2;for;x=0.50,;and;Al_5Fe_2,;and;Al_0.5Fe_0.5;for;x=0.75 are observed. After being annealed at 500circ;and;600circCfor 1 h for leached specimens, these non-equi-librium phases transformed into fcc Cu and CuFe_2O_4phases for the x=0.25 specimen, and into bcc alpha-Fe,;fcc;Cu,;and;CuFe_2O_4 phases for both the x=0.50 and the x=0.75 specimens. The saturation magnetization decreased with increasing milling time for Al_0.6(Fe_xCu_1-x)_0.4 alloy powders. On cooling the leached specimens from 800~850°C,;the magnetization first sharply increase at about 491.4°C,;745°C,;and;750.0°C for x=0.25, x=0.50, and x=0.75 specimens, repectively.
- [English]
- Chemical Leaching of Non-Equilibrium Al(Fe-Co) Powder Produced by Rod Milling
- Hyun-Goo Kim
- J Korean Powder Metall Inst. 2003;10(5):305-309.
- DOI: https://doi.org/10.4150/KPMI.2003.10.5.305

- 464 View
- 0 Download
-
 Abstract
Abstract
 PDF
PDF - We report on the formation and chemical leaching of non-equilibrium Al_0.6(Fe_75Co_25) alloy produced by rod milling. X-ray diffractometry, transmission electron microscopy, differential scanning calorimetry, scanning electron microscopy, and vibrating sample magnetometry were used to characterize the as-milled and leached specimens. After 400 h, only the Al_0.4Fe_0.6 peak of the body-centered cubic type was present in the XRD pattern. The entire rod milling process could be divided into three different stages of milling: agglomeration, disintegration, and homogenization. The saturation magnetization, M_s decreased with increased milling time, the M_s of the powders before milling was about 113.8 emu/g, the M_s after milling for 400 h was about 11.55 emu/g. Leaching of the Al in KOH of the Al at room temperature from the as-milled powders did not induce any significant change in the diffraction pattern. After the leached specimen had been annealed at 600°C for 1 hour, the nanoscale crystalline phases were transformed into the bcc Fe, cubic Co, and CoFe_2O_4 phases. On cooling the specimen from 850°C, the degree of magnetization increased slightly, then increased sharply at approximately 364.8°C, indicating that the bcc Al_0.4Fe_0.6 phase had been transformed to the Fe and Co phases.
TOP
 KPMI
KPMI

 First
First Prev
Prev


