Search
- Page Path
- HOME > Search
- [Korean]
- Synthesis and Investigation of LiVPO4O1-xFxvia Control of the Fluorine Content for Cathode of Lithium-ion Batteries
- Minkyung Kim, Dong-hee Lee, Changyu Yeo, Sooyeon Choi, Chiwon Choi, Hyunmin Yoon
- J Powder Mater. 2023;30(6):516-520. Published online December 1, 2023
- DOI: https://doi.org/10.4150/KPMI.2023.30.6.516
- 1,021 View
- 19 Download
-
 Abstract
Abstract
 PDF
PDF Highly safe lithium-ion batteries (LIBs) are required for large-scale applications such as electrical vehicles and energy storage systems. A highly stable cathode is essential for the development of safe LIBs. LiFePO4 is one of the most stable cathodes because of its stable structure and strong bonding between P and O. However, it has a lower energy density than lithium transition metal oxides. To investigate the high energy density of phosphate materials, vanadium phosphates were investigated. Vanadium enables multiple redox reactions as well as high redox potentials. LiVPO4O has two redox reactions (V5+/V4+/V3+) but low electrochemical activity. In this study, LiVPO4O is doped with fluorine to improve its electrochemical activity and increase its operational redox potential. With increasing fluorine content in LiVPO4O1-xFx, the local vanadium structure changed as the vanadium oxidation state changed. In addition, the operating potential increased with increasing fluorine content. Thus, it was confirmed that fluorine doping leads to a strong inductive effect and high operating voltage, which helps improve the energy density of the cathode materials.
- [Korean]
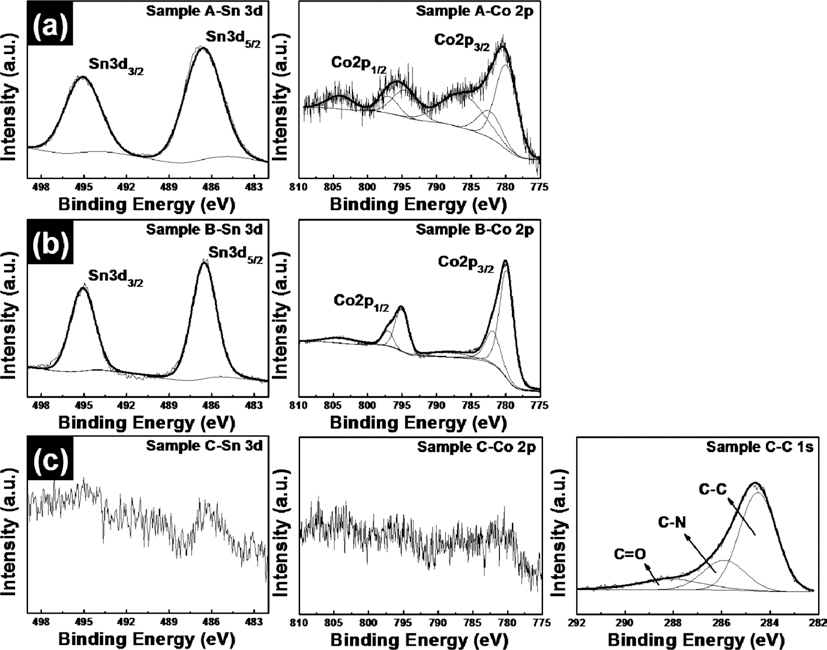
- Synthesis and Characterization of SnO2-CoO/carbon-coated CoO Core/shell Nanowire Composites
- Yu-Jin Lee, Bon-Ryul Koo, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2014;21(5):360-365. Published online October 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.5.360
- 720 View
- 0 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF SnO2-CoO/carbon-coated CoO core/shell nanowire composites were synthesized by using electrospinning and hydrothermal methods. In order to obtain SnO2-CoO/carbon-coated CoO core/shell nanowire composites, SnO2-Co3O4 nanowire composites and SnO2-Co3O4/polygonal Co3O4 core/shell nanowire composites are also synthesized. To demonstrate their structural, chemical bonding, and morphological properties, field-emission scanning electron microscopy, transmission electron microscopy, X-ray diffraction, and X-ray photoelectron spectroscopy were carried out. These results indicated that the morphologies and structures of the samples were changed from SnO2-Co3O4 nanowires having cylindrical structures to SnO2-Co3O4/Co3O4 core/shell nanowires having polygonal structures after a hydrothermal process. At last, SnO2-CoO/carbon-coated CoO core/shell nanowire composites having irregular and high surface area are formed after carbon coating using a polypyrrole (PPy). Also, there occur phases transformation of cobalt phases from Co3O4 to CoO during carbon coating using a PPy under a argon atmosphere.
-
Citations
Citations to this article as recorded by- Co-Embedded Graphitic Porous Carbon Nanofibers for Pt-Free Counter Electrode in Dye-Sensitized Solar Cells
혜란 안, 혜린 강, 효정 선, 지호 한, 효진 안
Korean Journal of Materials Research.2015; 25(12): 672~677. CrossRef - Synthesis of Perforated Polygonal Cobalt Oxides usinga Carbon Nanofiber Template
Dong-Yo Sin, Geon-Hyoung An, Hyo-Jin Ahn
Journal of Korean Powder Metallurgy Institute.2015; 22(5): 350. CrossRef
- Co-Embedded Graphitic Porous Carbon Nanofibers for Pt-Free Counter Electrode in Dye-Sensitized Solar Cells
- [Korean]
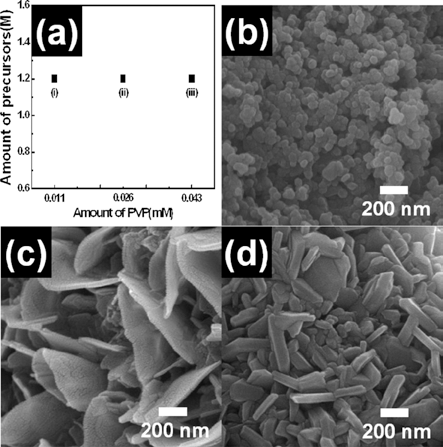
- Fabrication of Flake-like LiCoO2 Nanopowders using Electrospinning
- Bon-Ryul Koo, Geon-Hyoung An, Hyo-Jin Ahn
- J Korean Powder Metall Inst. 2014;21(2):108-113. Published online April 1, 2014
- DOI: https://doi.org/10.4150/KPMI.2014.21.2.108
- 807 View
- 4 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
PDF Flake-like LiCoO2 nanopowders were fabricated using electrospinning. To investigate their formation mechanism, field-emssion scanning electron microscopy, X-ray diffraction, and X-ray photoelectron spectroscopy were carried out. Among various parameters of electrospinning, we controlled the molar concentration of the precursor and the PVP polymer. When the molar concentration of lithium and cobalt was 0.45 M, the morphology of LiCoO2 nanopowders was irregular and round. For 1.27 M molar concentration, the LiCoO2 nanopowders formed with flake-like morphology. For the PVP polymer, the molar concentration was set to 0.011 mM, 0.026 mM, and 0.043 mM. Irregular LiCoO2 nanopowders were formed at low concentration (0.011 mM), while flake-like LiCoO2 were formed at high concentration (0.026 mM and 0.043 mM). Thus, optimized molar concentration of the precursor and the PVP polymer may be related to the successful formation of flake-like LiCoO2 nanopowders. As a results, the synthesized LiCoO2 nanopowder can be used as the electrode material of Li-ion batteries.
-
Citations
Citations to this article as recorded by- Electrochemical Behavior of Well-dispersed Catalysts on Ruthenium Oxide Nanofiber Supports
Geon-Hyoung An, Hyo-Jin Ahn
Journal of Korean Powder Metallurgy Institute.2017; 24(2): 96. CrossRef - Synthesis and Characterization of SnO2-CoO/carbon-coated CoO Core/shell Nanowire Composites
Yu-Jin Lee, Bon-Ryul Koo, Hyo-Jin Ahn
Journal of Korean Powder Metallurgy Institute.2014; 21(5): 360. CrossRef
- Electrochemical Behavior of Well-dispersed Catalysts on Ruthenium Oxide Nanofiber Supports
TOP
 KPMI
KPMI


 First
First Prev
Prev


