Search
- Page Path
- HOME > Search
- [Korean]
- Synthesis and analysis CdSe/ZnS quantum dot with a Core/shell Continuous Synthesis System Using a Microfluidic Reactor
- Myung Hwan Hong, So Young Joo, Lee-Seung Kang, Chan Gi Lee
- J Korean Powder Metall Inst. 2018;25(2):132-136. Published online April 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.2.132
- 1,933 View
- 10 Download
-
 Abstract
Abstract
 PDF
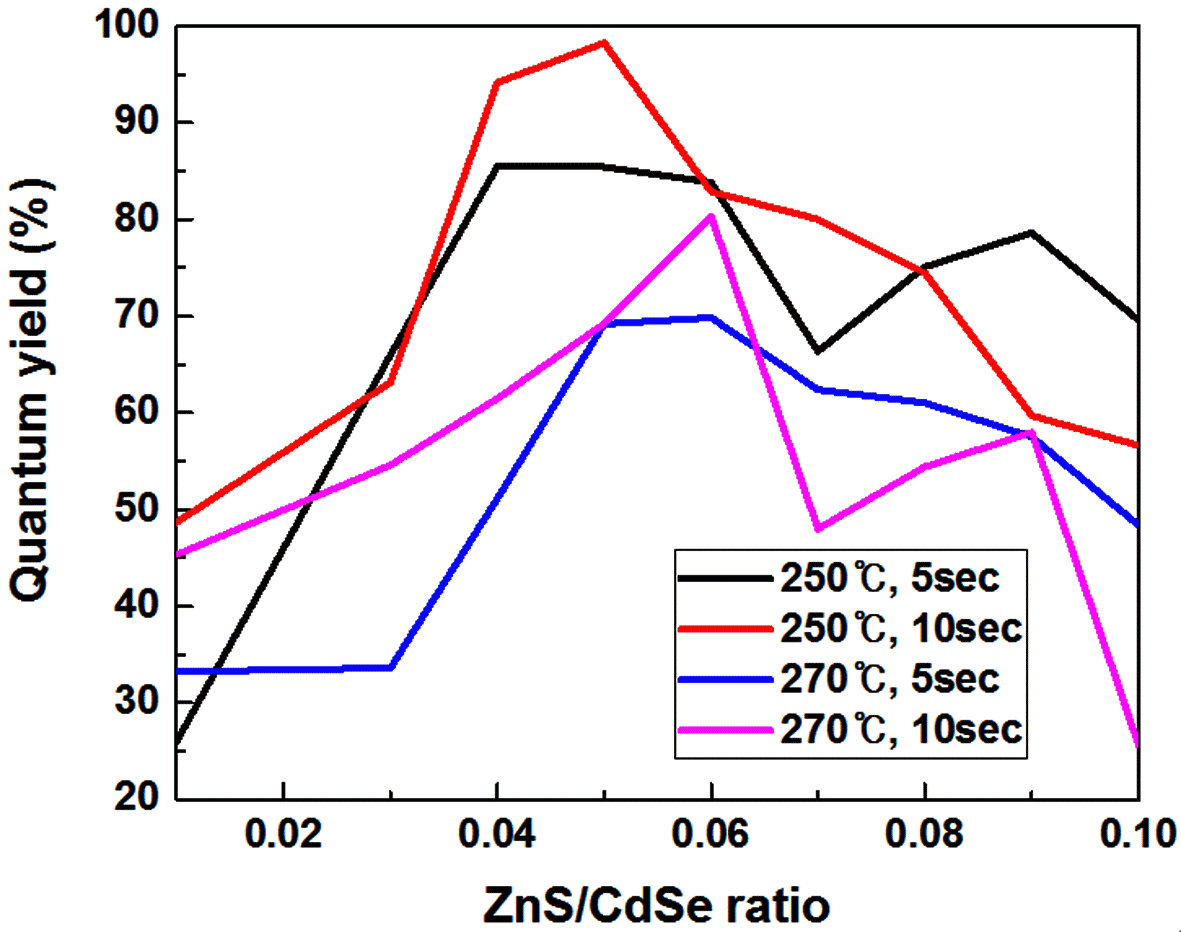
PDF Core/shell CdSe/ZnS quantum dots (QDs) are synthesized by a microfluidic reactor-assisted continuous reactor system. Photoluminescence and absorbance of synthesized CdSe/ZnS core/shell QDs are investigated by fluorescence spectrophotometry and online UV-Vis spectrometry. Three reaction conditions, namely; the shell coating reaction temperature, the shell coating reaction time, and the ZnS/CdSe precursor volume ratio, are combined in the synthesis process. The quantum yield of the synthesized CdSe QDs is determined for each condition. CdSe/ZnS QDs with a higher quantum yield are obtained compared to the discontinuous microfluidic reactor synthesis system. The maximum quantum efficiency is 98.3% when the reaction temperature, reaction time, and ZnS/CdSe ratio are 270°C, 10 s, and 0.05, respectively. Obtained results indicate that a continuous synthesis of the Core/shell CdSe/ZnS QDs with a high quantum efficiency could be achieved by isolating the reaction from the external environment.
- [Korean]
- Synthesis and Properties of InP/ZnS core/shell Nanoparticles with One-pot process
- So Yeong Joo, Myung Hwan Hong, Leeseung Kang, Tae Hyung Kim, Chan Gi Lee
- J Korean Powder Metall Inst. 2017;24(1):11-16. Published online February 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.1.11
- 1,037 View
- 9 Download
-
 Abstract
Abstract
 PDF
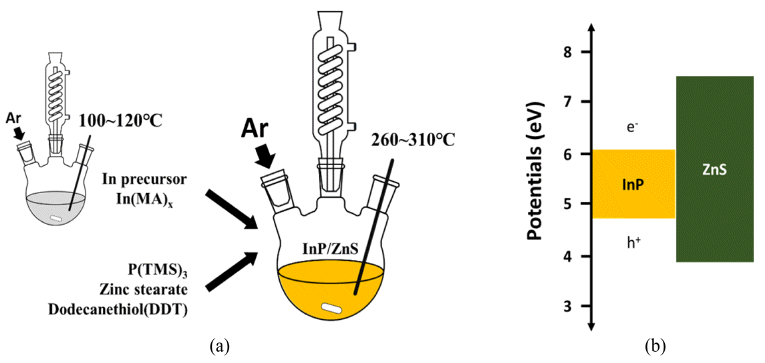
PDF In this study, simple chemical synthesis of green emitting Cd-free InP/ZnS QDs is accomplished by reacting In, P, Zn, and S precursors by one-pot process. The particle size and the optical properties were tailored, by controlling various experimental conditions, including [In]/[MA] (MA: myristic acid) mole ratio, reaction temperature and reaction time. The results of ultraviolet–visible spectroscopy (UV-vis), and of photoluminescence (PL), reveal that the exciton emission of InP was improved by surface coating, with a layer of ZnS. We report the correlation between each experimental condition and the luminescent properties of InP/ZnS core/shell QDs. Transmission electron microscopy (TEM), and X-ray powder diffraction (XRD) techniques were used to characterize the as-synthesized QDs. In contrast to core nanoparticles, InP/ZnS core/shell treated with surface coating shows a clear ultraviolet peak. Besides this work, we need to study what clearly determines the shell kinetic growth mechanism of InP/ZnS core shell QDs.
- [Korean]
- Synthesis and analysis CdSe Quantum dot with a Microfluidic Reactor Using a Combinatorial Synthesis System
- Myung Hwan Hong, Duk-Hee Lee, Lee-Seung Kang, Chan Gi Lee, Bum-Sung Kim, Nam-Hoon Kim
- J Korean Powder Metall Inst. 2016;23(2):143-148. Published online April 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.2.143
- 882 View
- 7 Download
-
 Abstract
Abstract
 PDF
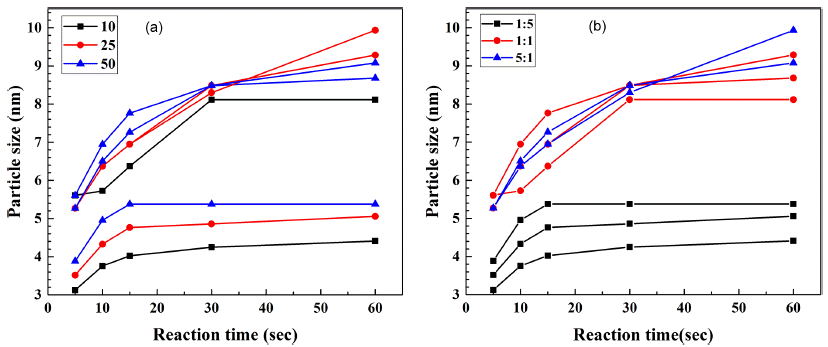
PDF A microfluidic reactor with computer-controlled programmable isocratic pumps and online detectors is employed as a combinatorial synthesis system to synthesize and analyze materials for fabricating CdSe quantum dots for various applications. Four reaction condition parameters, namely, the reaction temperature, reaction time, Cd/Se compositional ratio, and precursor concentration, are combined in synthesis condition sets, and the size of the synthesized CdSe quantum dots is determined for each condition. The average time corresponding to each reaction condition for obtaining the ultraviolet–visible absorbance and photoluminescence spectra is approximately 10 min. Using the data from the combinatorial synthesis system, the effects of the reaction conditions on the synthesized CdSe quantum dots are determined. Further, the data is used to determine the relationships between the reaction conditions and the CdSe particle size. This method should aid in determining and selecting the optimal conditions for synthesizing nanoparticles for diverse applications.
TOP
 KPMI
KPMI

 First
First Prev
Prev


