Search
- Page Path
- HOME > Search
- [Korean]
- Study on the Recovery Silver and Nanoparticles Synthesis from LTCC By-products of Lowly Concentrated Silver
- Soyeong Joo, Nak-Kyoon Ahn, Chan Gi Lee, Jin-Ho Yoon
- J Korean Powder Metall Inst. 2018;25(3):232-239. Published online June 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.3.232
- 816 View
- 4 Download
-
 Abstract
Abstract
 PDF
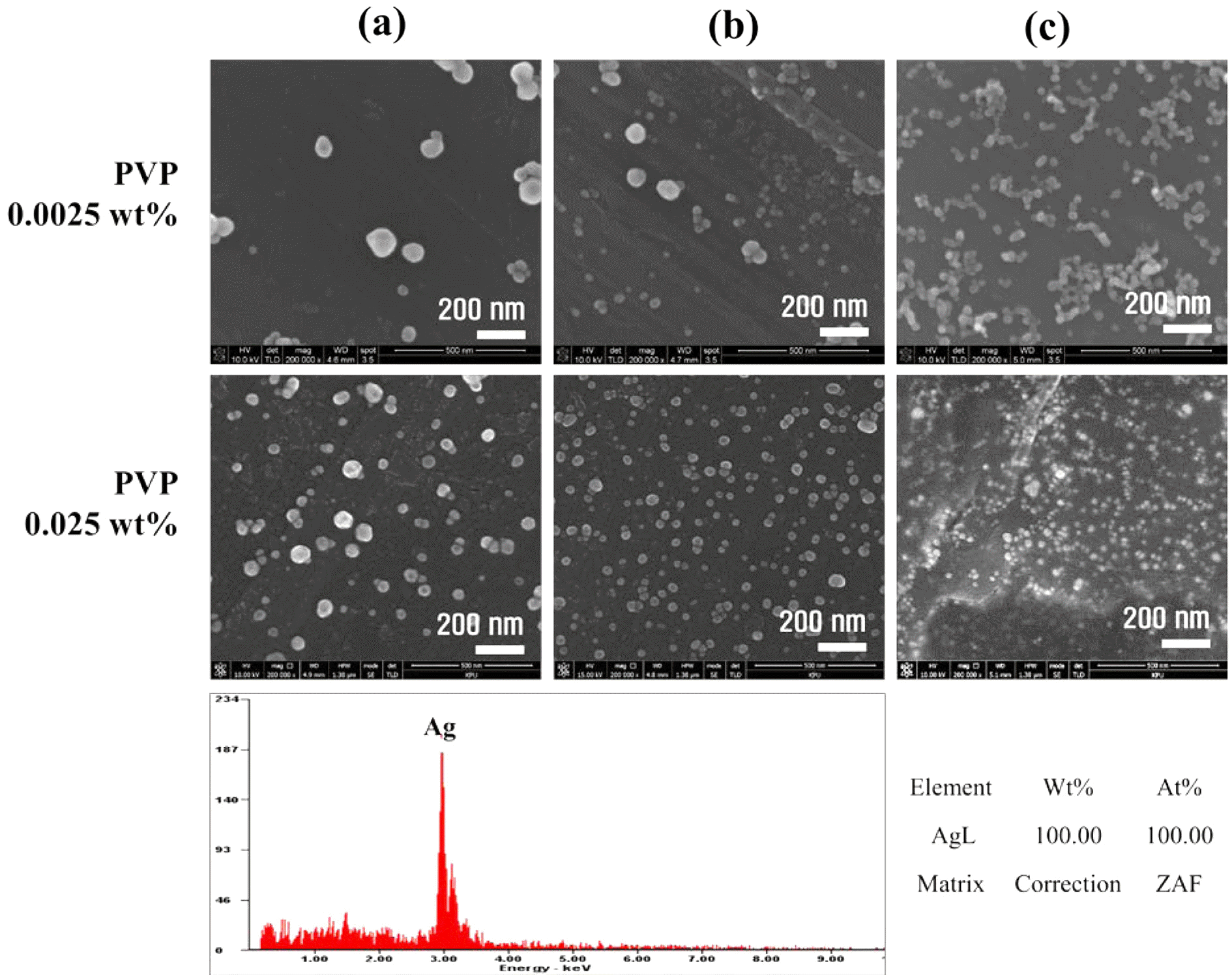
PDF In this paper, the recovery and nanoparticle synthesis of Ag from low temperature co-fired ceramic (LTCC) by-products are studied. The effect of reaction behavior on Ag leaching conditions from the LTCC by-products is confirmed. The optimum leaching conditions are determined to be: 5 M HNO3, a reaction temperature of 75°C, and a pulp density of 50 g/L at 60 min. For the selective recovery of Ag, the [Cl]/[Ag] equivalence ratio experiment is performed using added HCl; most of the Ag (more than 99%) is recovered. The XRD and MP-AES results confirm that the powder is AgCl and that impurities are at less than 1%. Ag nanoparticles are synthesized using a chemical reduction process for recycling, NaBH4 and PVP are used as reducing agents and dispersion stabilizers. UV-vis and FE-SEM results show that AgCl powder is precipitated and that Ag nanoparticles are synthesized. Ag nanoparticles of 100% Ag are obtained under the chemical reaction conditions.
- [Korean]
- A Study on the Preparation of Rare Earth Oxide Powder for Rare Earth Precipitates Recovered from Spent Ni-MH Batteries
- Dae-Weon Kim, Nak-Kyoon Ahn, Hyun-Woo Shim, Kyung-Soo Park, Hee-Lack Choi
- J Korean Powder Metall Inst. 2018;25(3):213-219. Published online June 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.3.213
- 562 View
- 2 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
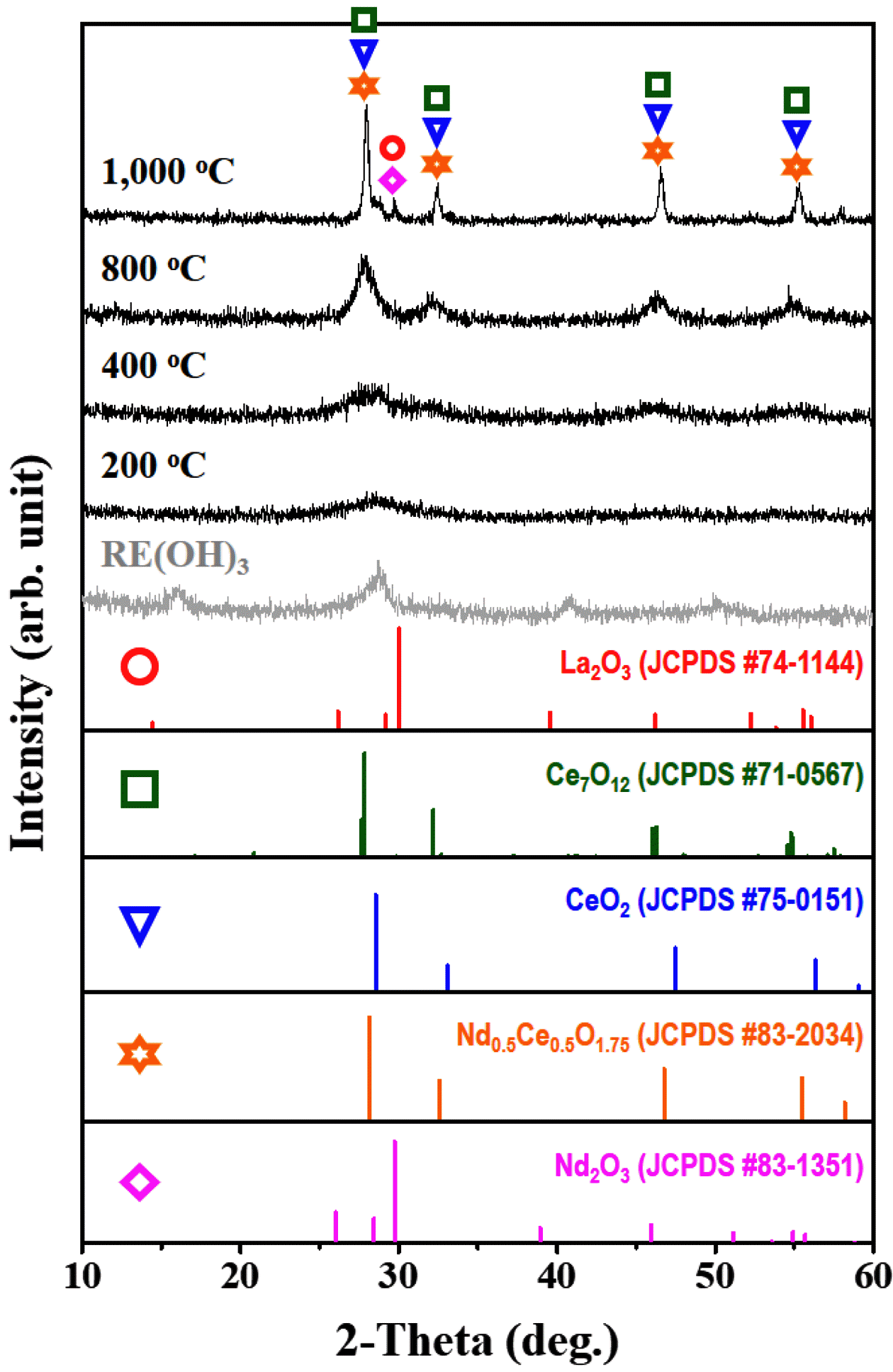
PDF We report a method for preparing rare earth oxides (RexOy) from the recycling process for spent Ni-metal hydride (Ni-MH) batteries. This process first involves a leaching of spent Ni-MH powders with sulfuric acid at 90°C, resulting in rare earth precipitates (i.e., NaRE(SO4)2·H2O, RE = La, Ce, Nd), which are converted into rare earth oxides via two different approaches: i) simple heat treatment in air, and ii) metathesis reaction with NaOH at 70°C. Not only the morphological features but also the crystallographic structures of all products are systematically investigated using field-emission scanning electron microscopy (FESEM) and X-ray diffraction (XRD); their thermal behaviors are also analyzed. In particular, XRD results show that some of the rare earth precipitates are converted into oxide form (such as La2O3, Ce2O3, and Nd2O3) with heat treatment at 1200°C; however, secondary peaks are also observed. On the other hand, rare earth oxides, RexOy can be successfully obtained after metathesis of rare earth precipitates, followed by heat treatment at 1000°C in air, along with a change of crystallographic structures, i.e., NaRE(SO4)2·H2O → RE(OH)3 →RexOy.
-
Citations
Citations to this article as recorded by- Recycling of Rechargeable Batteries: Insights from a Bibliometrics‐Based Analysis of Emerging Publishing and Research Trends
Jiao Lin, Xiaodong Zhang, Li Cai, Ersha Fan, Shumeng Wu, Su Ma, Feng Wu, Renjie Chen, Li Li
Advanced Energy and Sustainability Research.2022;[Epub] CrossRef -
Synthesis of Rare Earth Oxide from NaREE(SO4)2·H2O by Ion Substitution Reaction
Dae-Weon Kim, Bo-Ram Kim, Hee-Lack Choi
Journal of the Korean Society of Mineral and Energy Resources Engineers.2020; 57(5): 405. CrossRef
- Recycling of Rechargeable Batteries: Insights from a Bibliometrics‐Based Analysis of Emerging Publishing and Research Trends
TOP
 KPMI
KPMI


 First
First Prev
Prev


