Search
- Page Path
- HOME > Search
- [Korean]
- Characteristics of WO3-CuO Powder Mixture Prepared by High-Energy Ball Milling in a Bead Mill for the Synthesis of W-Cu Nanocomposite Powder
- Hae-Ryong Park, Sung-Soo Ryu
- J Korean Powder Metall Inst. 2017;24(5):406-413. Published online October 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.5.406
- 1,031 View
- 5 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
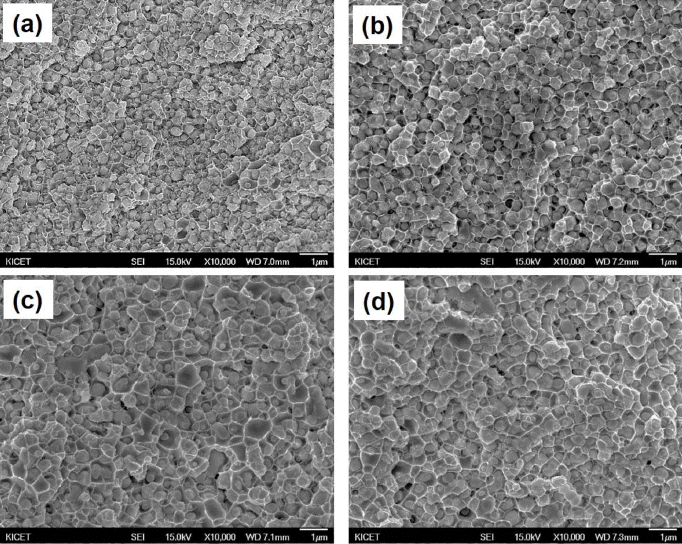
PDF A Nanosized WO3 and CuO powder mixture is prepared using novel high-energy ball milling in a bead mill to obtain a W-Cu nanocomposite powder, and the effect of milling time on the structural characteristics of WO3-CuO powder mixtures is investigated. The results show that the ball-milled WO3-CuO powder mixture reaches at steady state after 10 h milling, characterized by the uniform and narrow particle size distribution with primary crystalline sizes below 50 nm, a specific surface area of 37 m2/g, and powder mean particle size (D50) of 0.57 μm. The WO3-CuO powder mixtures milled for 10 h are heat-treated at different temperatures in H2 atmosphere to produce W-Cu powder. The XRD results shows that both the WO3 and CuO phases can be reduced to W and Cu phases at temperatures over 700°C. The reduced W-Cu nanocomposite powder exhibits excellent sinterability, and the ultrafine W-Cu composite can be obtained by the Cu liquid phase sintering process.
-
Citations
Citations to this article as recorded by- Morphological Characteristics of W/Cu Composite Nanoparticles with Complex Phase Structure Synthesized via Reactive Radio Frequency (RF) Thermal Plasma
Chulwoong Han, Song-Yi Kim, Soobin Kim, Ji-Woon Lee
Metals.2024; 14(9): 1070. CrossRef
- Morphological Characteristics of W/Cu Composite Nanoparticles with Complex Phase Structure Synthesized via Reactive Radio Frequency (RF) Thermal Plasma
- [Korean]
- Fabrication of Fe3O4/Fe/Graphene nanocomposite powder by Electrical Wire Explosion in Liquid Media and its Electrochemical Properties
- Yoo-Young Kim, Ji-Seub Choi, Hoi-Jin Lee, Kwon-Koo Cho
- J Korean Powder Metall Inst. 2017;24(4):308-314. Published online August 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.4.308
- 1,009 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
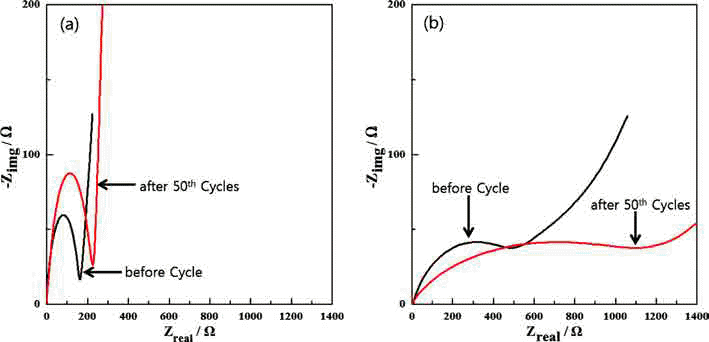
PDF Fe3O4/Fe/graphene nanocomposite powder is synthesized by electrical wire explosion of Fe wire and dispersed graphene in deionized water at room temperature. The structural and electrochemical characteristics of the powder are characterized by the field-emission scanning electron microscopy, X-ray diffraction, Raman spectroscopy, field-emission transmission electron microscopy, cyclic voltammetry, and galvanometric discharge-charge method. For comparison, Fe3O4/Fe nanocomposites are fabricated under the same conditions. The Fe3O4/Fe nanocomposite particles, around 15-30 nm in size, are highly encapsulated in a graphene matrix. The Fe3O4/Fe/graphene nanocomposite powder exhibits a high initial charge specific capacity of 878 mA/g and a high capacity retention of 91% (798 mA/g) after 50 cycles. The good electrochemical performance of the Fe3O4/Fe/graphene nanocomposite powder is clearly established by comparison of the results with those obtained for Fe3O4/Fe nanocomposite powder and is attributed to alleviation of volume change, good distribution of electrode active materials, and improved electrical conductivity upon the addition of graphene.
-
Citations
Citations to this article as recorded by- Preparation of magnetic metal and graphene hybrids with tunable morphological, structural and magnetic properties
Kyunbae Lee, Joonsik Lee, Byung Mun Jung, Byeongjin Park, Taehoon Kim, Sang Bok Lee
Applied Surface Science.2019; 478: 733. CrossRef
- Preparation of magnetic metal and graphene hybrids with tunable morphological, structural and magnetic properties
TOP
 KPMI
KPMI


 First
First Prev
Prev


