Search
- Page Path
- HOME > Search
- [Korean]
- The Effects of Hexamethylenetetramine Concentration on the Structural and Electrochemical Performances of Ni(OH)2 Powder for Pseudocapacitor Applications
- Dong Yeon Kim, Young-Min Jeong, Seong-Ho Baek, Injoon Son
- J Korean Powder Metall Inst. 2019;26(3):231-236. Published online June 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.3.231
- 1,178 View
- 2 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
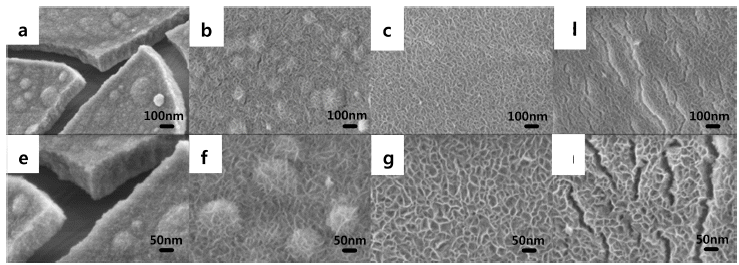
PDF Ni hydroxides (Ni(OH)2) are synthesized on Ni foam by varying the hexamethylenetetramine (HMT) concentration using an electrodeposition process for pseudocapacitor (PC) applications. In addition, the effects of HMT concentration on the Ni(OH)2 structure and the electrochemical properties of the PCs are investigated. HMT is the source of amine-based OH− in the solution; thus, the growth rate and morphological structure of Ni(OH)2 are influenced by HMT concentration. When Ni(OH)2 is electrodeposited at a constant voltage mode of -0.85 V vs. Ag/AgCl, the cathodic current and the number of nucleations are significantly reduced with increasing concentration of HMT from 0 to 10 mM. Therefore, Ni(OH)2 is sparsely formed on the Ni foam with increasing HMT concentration, showing a layered double-hydroxide structure. However, loosely packed Ni(OH)2 grains that are spread on Ni foam maintain a much greater surface area for reaction and result in the effective utilization of the electrode material due to the steric hindrance effect. It is suggested that the Ni(OH)2 electrodes with HMT concentration of 7.5 mM have the maximum specific capacitance (1023 F/g), which is attributed to the facile electrolyte penetration and fast proton exchange via optimized surface areas.
-
Citations
Citations to this article as recorded by- Review of Domestic Research Trends on Layered Double Hydroxide (LDH) Materials: Based on Research Articles in Korean Citation Index (KCI)
Seon Yong Lee, YoungJae Kim, Young Jae Lee
Economic and Environmental Geology.2023; 56(1): 23. CrossRef
- Review of Domestic Research Trends on Layered Double Hydroxide (LDH) Materials: Based on Research Articles in Korean Citation Index (KCI)
- [Korean]
- Preparation of Ni(OH)2 Hollow Spheres by Solvent Displacement Crystallization Using Micro-Injection Device
- Seiki Kim, Kyungsoo Park, Kwang-Il Jung
- J Korean Powder Metall Inst. 2016;23(4):311-316. Published online August 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.4.311
- 1,040 View
- 9 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
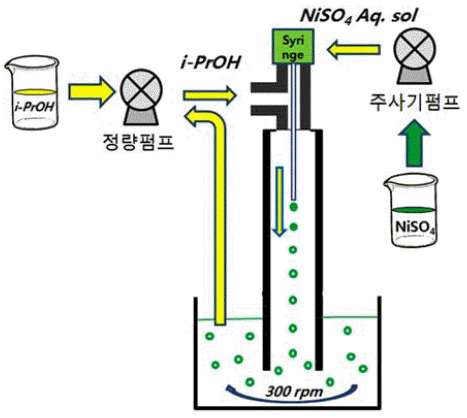
PDF Ni(OH)2 hollow spheres have been prepared by solvent displacement crystallization using a micro-injection device, and the effect of process parameters such as concentration and the relative ratio of the injection speed of the precursor solution, which is an aqueous solution of NiSO4·6H2O, to isopropyl alcohol of displacement solvent have been investigated. The crystal phases after NaOH treatment are in the β-phase for all process parameters. A higher concentration of NiSO4·6H2O aqueous solution is injected by a micro-injection device and bigger Ni(OH)2 hollow spheres with a narrower particle size distribution are formed. The crystallinity and hardness of the as-obtained powder are so poor that hydrothermal treatment of the as-obtained Ni(OH)2 at 120°C for 24 h in distilled water is performed in order to greatly improve the crystallinity. It is thought that a relative ratio of the injection speed of NiSO4·6H2O to that of isopropyl alcohol of at least more than 1 is preferable to synthesize Ni(OH)2 hollow spheres. It is confirmed that this solution- based process is very effective in synthesizing ceramic hollow spheres by simple adjustment of the process parameters such as the concentration and the injection speed.
-
Citations
Citations to this article as recorded by- The Effects of Hexamethylenetetramine Concentration on the Structural and Electrochemical Performances of Ni(OH)2 Powder for Pseudocapacitor Applications
Dong Yeon Kim, Young-Min Jeong, Seong-Ho Baek, Injoon Son
Journal of Korean Powder Metallurgy Institute.2019; 26(3): 231. CrossRef
- The Effects of Hexamethylenetetramine Concentration on the Structural and Electrochemical Performances of Ni(OH)2 Powder for Pseudocapacitor Applications
TOP
 KPMI
KPMI


 First
First Prev
Prev


