Search
- Page Path
- HOME > Search
- [Korean]
- Effect of TiO2 Content on High-Temperature Degradation Behavior of Nd2O3 and Yb2O3 Doped YSZ Composite Materials
- Gye-Won Lee, Seonung Choi, Tae-jun Park, Jong-il Kim, In-hwan Lee, Yoon-seok Oh
- J Powder Mater. 2024;31(5):431-436. Published online October 31, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00269
- 656 View
- 12 Download
-
 Abstract
Abstract
 PDF
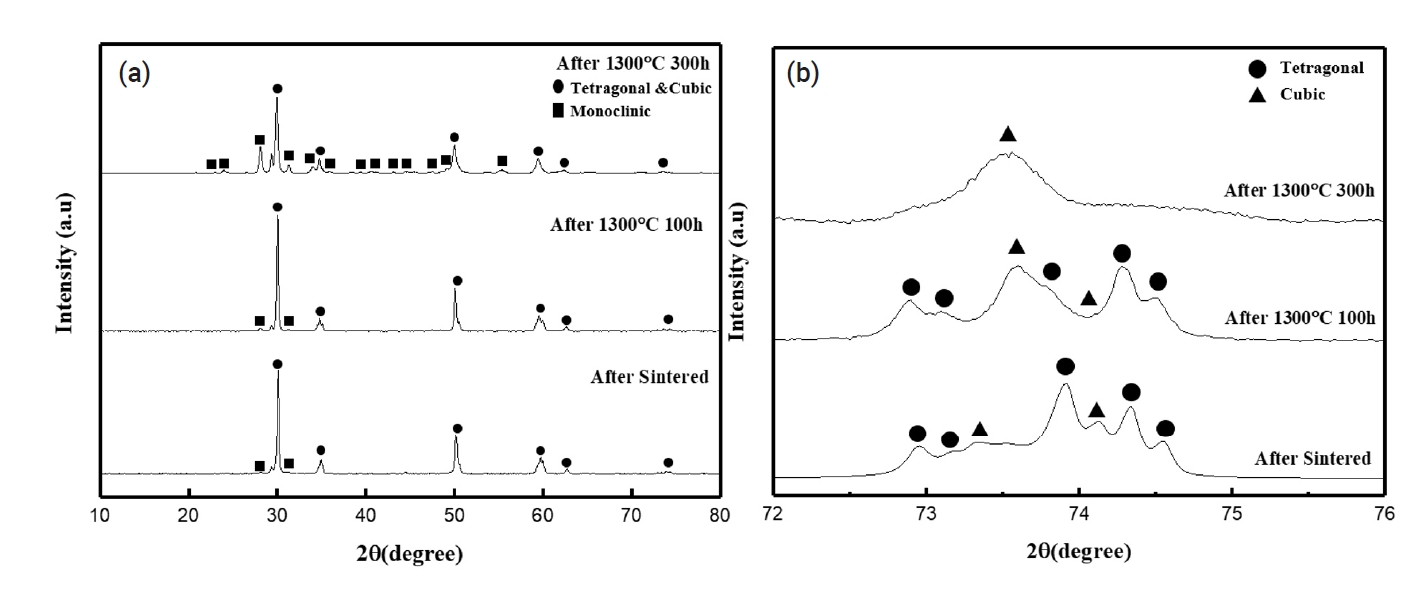
PDF - Hot section components of gas turbines are exposed to a high operating temperature environment. To protect these components, thermal barrier coatings (TBC) are applied to their surfaces. Yttria-stabilized zirconia (YSZ), which is widely used as a TBC material, faces limitations at temperatures above 1200℃. To mitigate these issues, research has focused on adding lanthanide rare earth oxides and tetravalent oxides to prevent the phase-transformation of the monoclinic phase in zirconia. This study investigated the effects of varying TiO2 content in Nd2O3 and Yb2O3 co-doped YSZ composites. Increasing TiO2 content effectively suppressed formation of the monoclinic phase and increased the thermal degradation resistance compared to YSZ in environments over 1200℃. These findings will aid in developing more thermally stable and efficient TBC materials for application in high-temperature environments.
- [Korean]
- Prediction the Phase Transformation Time of Binary Alloy System by calculating the Input Energy of Mechanical Alloying
- Dong-Kyu Park, In-Shup Ahn
- J Korean Powder Metall Inst. 2019;26(2):107-111. Published online April 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.2.107
- 603 View
- 1 Download
-
 Abstract
Abstract
 PDF
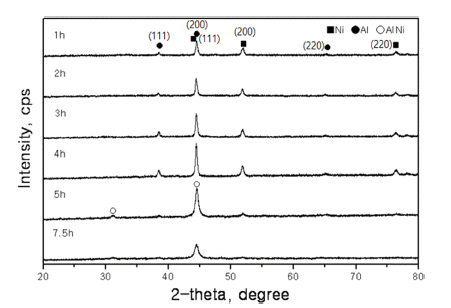
PDF The activation energy to create a phase transformation or for the reaction to move to the next stage in the milling process can be calculated from the slop of the DSC plot, obtained at the various heating rates for mechanically activated Al-Ni alloy systems by using Kissinger’s equation. The mechanically activated material has been called “the driven material” as it creates new phases or intermetallic compounds of AlNi in Al-Ni alloy systems. The reaction time for phase transformation by milling can be calculated using the activation energy obtained from the above mentioned method and from the real required energy. The real required energy (activation energy) could be calculated by subtracting the loss energy from the total input energy (calculated input energy from electric motor). The loss energy and real required energy divided by the reaction time are considered the “metabolic energy” and “the effective input energy”, respectively. The milling time for phase transformation at other Al-Co alloy systems from the calculated data of Al-Ni systems can be predicted accordingly.
- [Korean]
- Study on thermal behavior of Ammonium Hexafluofide Titanate for Synthesis of TiO2 Powders
- Duk-Hee Lee, Jae-Ryang Park, Chan-Gi Lee, Kyung-Soo Park, Hyeon-Mo Kim
- J Korean Powder Metall Inst. 2016;23(5):353-357. Published online October 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.5.353
- 603 View
- 1 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
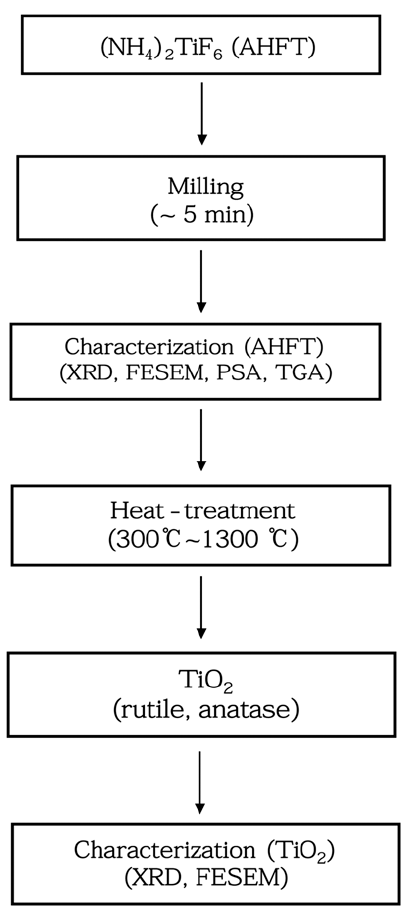
PDF In this study, TiO2 powders are synthesized from ammonium hexafluoride titanate (AHFT, (NH4)2TiF6) as a precursor by heat treatment. First, we evaluate the physical properties of AHFT using X-ray diffraction (XRD), particle size analysis (PSA), thermogravimetric analysis (TGA), and field-emission scanning electron microscopy (FESEM). Then, to prepare the TiO2 powders, is heat-treated at 300-1300°C for 1 h. The ratio of anatase to rutile phase in TiO2 is estimated by XRD. The anatase phase forms at 500°C and phase transformation to the rutile phase occurs at 1200°C. Increase in the particle size is observed upon increasing the reaction temperature, and the phase ratio of the rutile phase is determined from a comparison with the calculated XRD data. Thus, we show that anatase and rutile TiO2 powders could be synthesized using AHFT as a raw material, and the obtained data are utilized for developing a new process for producing high-quality TiO2 powder.
-
Citations
Citations to this article as recorded by- Photocatalytic activity of rutile TiO2 powders coupled with anatase TiO2 nanoparticles using surfactant
Jong Min Byun, Chun Woong Park, Young In Kim, Young Do Kim
journal of Korean Powder Metallurgy Institute.2018; 25(3): 257. CrossRef
- Photocatalytic activity of rutile TiO2 powders coupled with anatase TiO2 nanoparticles using surfactant
TOP
 KPMI
KPMI


 First
First Prev
Prev


