Search
- Page Path
- HOME > Search
- [Korean]
- Synthesis and Optical Property of (GaN)1-x(ZnO)x Nanoparticles Using an Ultrasonic Spray Pyrolysis Process and Subsequent Chemical Transformation
- Jeong Hyun Kim, Cheol-Hui Ryu, Myungjun Ji, Yomin Choi, Young-In Lee
- J Korean Powder Metall Inst. 2021;28(2):143-149. Published online April 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.2.143
- 482 View
- 2 Download
-
 Abstract
Abstract
 PDF
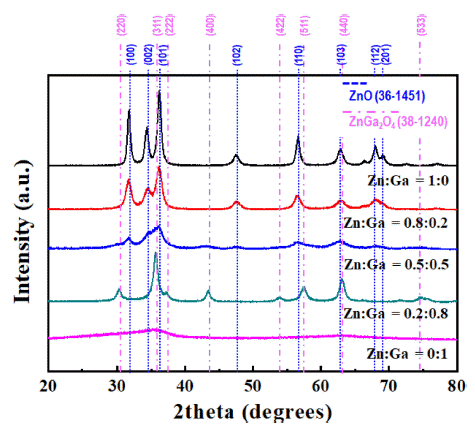
PDF In this study, (GaN)1-x(ZnO)x solid solution nanoparticles with a high zinc content are prepared by ultrasonic spray pyrolysis and subsequent nitridation. The structure and morphology of the samples are investigated by X-ray diffraction (XRD), field-emission scanning electron microscopy, and energy-dispersive X-ray spectroscopy. The characterization results show a phase transition from the Zn and Ga-based oxides (ZnO or ZnGa2O4) to a (GaN)1-x (ZnO)x solid solution under an NH3 atmosphere. The effect of the precursor solution concentration and nitridation temperature on the final products are systematically investigated to obtain (GaN)1-x(ZnO)x nanoparticles with a high Zn concentration. It is confirmed that the powder synthesized from the solution in which the ratio of Zn and Ga was set to 0.8:0.2, as the initial precursor composition was composed of about 0.8-mole fraction of Zn, similar to the initially set one, through nitriding treatment at 700°C. Besides, the synthesized nanoparticles exhibited the typical XRD pattern of (GaN)1-x(ZnO)x, and a strong absorption of visible light with a bandgap energy of approximately 2.78 eV, confirming their potential use as a hydrogen production photocatalyst.
- [Korean]
- Synthesis and Optical Property of TiO2 Nanoparticles Using a Salt-assisted Ultrasonic Spray Pyrolysis Process
- Myeong-Jun Ji, Woo-Young Park, Jae-Hyun Yoo, Young-In Lee
- J Korean Powder Metall Inst. 2019;26(1):34-39. Published online February 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.1.34
- 556 View
- 1 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Current synthesis processes for titanium dioxide (TiO2) nanoparticles require expensive precursors or templates as well as complex steps and long reaction times. In addition, these processes produce highly agglomerated nanoparticles. In this study, we demonstrate a simple and continuous approach to synthesize TiO2 nanoparticles by a salt-assisted ultrasonic spray pyrolysis method. We also investigate the effect of salt content in a precursor solution on the morphology and size of synthesized products. The synthesized TiO2 nanoparticles are systematically characterized by X-ray diffraction, transmission electron micrograph, and UV-Vis spectroscopy. These nanoparticles appear to have a single anatase phase and a uniform particle-size distribution with an average particle size of approximately 10 nm. By extrapolating the plots of the transformed Kubelka-Munk function versus the absorbed light energy, we determine that the energy band gap of the synthesized TiO2 nanoparticles is 3.25 eV.
-
Citations
Citations to this article as recorded by- Effect of Pyrolysis temperature on TiO2 Nanoparticles Synthesized by a Salt-assisted Ultrasonic Spray Pyrolysis Process
Jae-Hyun Yoo, Myeong-Jun Ji, Woo-Young Park, Young-In Lee
Journal of Korean Powder Metallurgy Institute.2019; 26(3): 237. CrossRef
- Effect of Pyrolysis temperature on TiO2 Nanoparticles Synthesized by a Salt-assisted Ultrasonic Spray Pyrolysis Process
TOP
 kpmi
kpmi


 First
First Prev
Prev


