Search
- Page Path
- HOME > Search
- [Korean]
- Preparation of Sintering Aid for Li7La3Zr2O12 Solid Electrolyte by Heat-treatment of Polymeric Precursors Containing Li and B
- Ran-Hee Shin, Sung-Soo Ryu
- J Korean Powder Metall Inst. 2018;25(2):151-157. Published online April 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.2.151

- 988 View
- 1 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF In this study, the compound Li3BO3 (LBO) is intended to be prepared by a polymeric complex method as a sintering aid for the densification of Li7La3Zr2O12 (LLZ) solid electrolyte. A polymeric precursor containing Li and B is heat-treated in an air atmosphere at a temperature range between 600°C and 800°C. Instead of LBO, the compound Li2+xC1-xBxO3 (LCBO) is unexpectedly synthesized after a heat-treatment of 700°C. The effect of LCBO addition on sintering behavior and ion conductivity of LLZ is studied. It is found that the LCBO compound could lead to significant improvements in the densification and ionic conductivity of LLZ compared to pure LLZ. After sintering at 1100°C, the density of the LLZ-12wt%LBO composite is 3.72 g/cm3, with a high Li-ion conductivity of 1.18 × 10−4 Scm-1 at 28°C, while the pure LLZ specimen had a densify of 2.98 g/cm3 and Li-ion conductivity of 5.98 × 10−6 Scm-1.
-
Citations
Citations to this article as recorded by- Characterization of Li1.5Al0.5Ge1.5(PO4)3 Solid Electrolyte with an Added Sintering Aid
Hyun-Joon Lee, Liyu-Liu, Won-Jong Jeong, Seoung-Ki Lee, Bong-Ki Ryu
Electronic Materials Letters.2023; 19(1): 55. CrossRef
- Characterization of Li1.5Al0.5Ge1.5(PO4)3 Solid Electrolyte with an Added Sintering Aid
- [Korean]
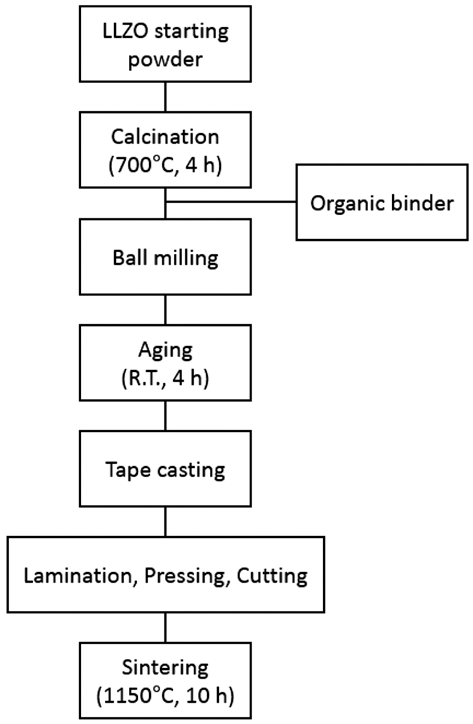
- Fabrication of Solid State Electrolyte Li7La3Zr2O12 thick Film by Tape Casting
- Ran-Hee Shin, Samick Son, Sung-Soo Ryu, Hyung-Tae Kim, Yoon-Soo Han
- J Korean Powder Metall Inst. 2016;23(5):379-383. Published online October 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.5.379
- 1,056 View
- 11 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF A thick film of Li7La3Zr2O12 (LLZO) solid-state electrolyte is fabricated using the tape casting process and is compared to a bulk specimen in terms of the density, microstructure, and ion conductivity. The final thickness of LLZO film after sintering is 240 μm which is stacked up with four sheets of LLZO green films including polymeric binders. The relative density of the LLZO film is 83%, which is almost the same as that of the bulk specimen. The ion conductivity of a LLZO thick film is 2.81 × 10−4 S/cm, which is also similar to that of the bulk specimen, 2.54 × 10−4 S/ cm. However, the microstructure shows a large difference in the grain size between the thick film and the bulk specimen. Although the grain boundary area is different between the thick film and the bulk specimen, the fact that both the ion conductivities are very similar means that no secondary phase exists at the grain boundary, which is thought to originate from nonstoichiometry or contamination.
-
Citations
Citations to this article as recorded by- Waste minimization in all-solid-state battery production via re-lithiation of the garnet solid electrolyte LLZO
Vivien Kiyek, Martin Hilger, Melanie Rosen, Jürgen Peter Gross, Markus Mann, Dina Fattakhova-Rohlfing, Ruth Schwaiger, Martin Finsterbusch, Olivier Guillon
Journal of Power Sources.2024; 609: 234709. CrossRef - Powder Aerosol Deposition as a Method to Produce Garnet‐Type Solid Ceramic Electrolytes: A Study on Electrochemical Film Properties and Industrial Applications
Tobias Nazarenus, Yanyan Sun, Jörg Exner, Jaroslaw Kita, Ralf Moos
Energy Technology.2021;[Epub] CrossRef - Synthesize of Nd2Fe14B Powders from 1-D Nd2Fe14B Wires using Electrospinning Process
Nu Si A Eom, Su Noh, Muhammad Aneeq Haq, Bum Sung Kim
Journal of Korean Powder Metallurgy Institute.2019; 26(6): 477. CrossRef
- Waste minimization in all-solid-state battery production via re-lithiation of the garnet solid electrolyte LLZO
- [Korean]
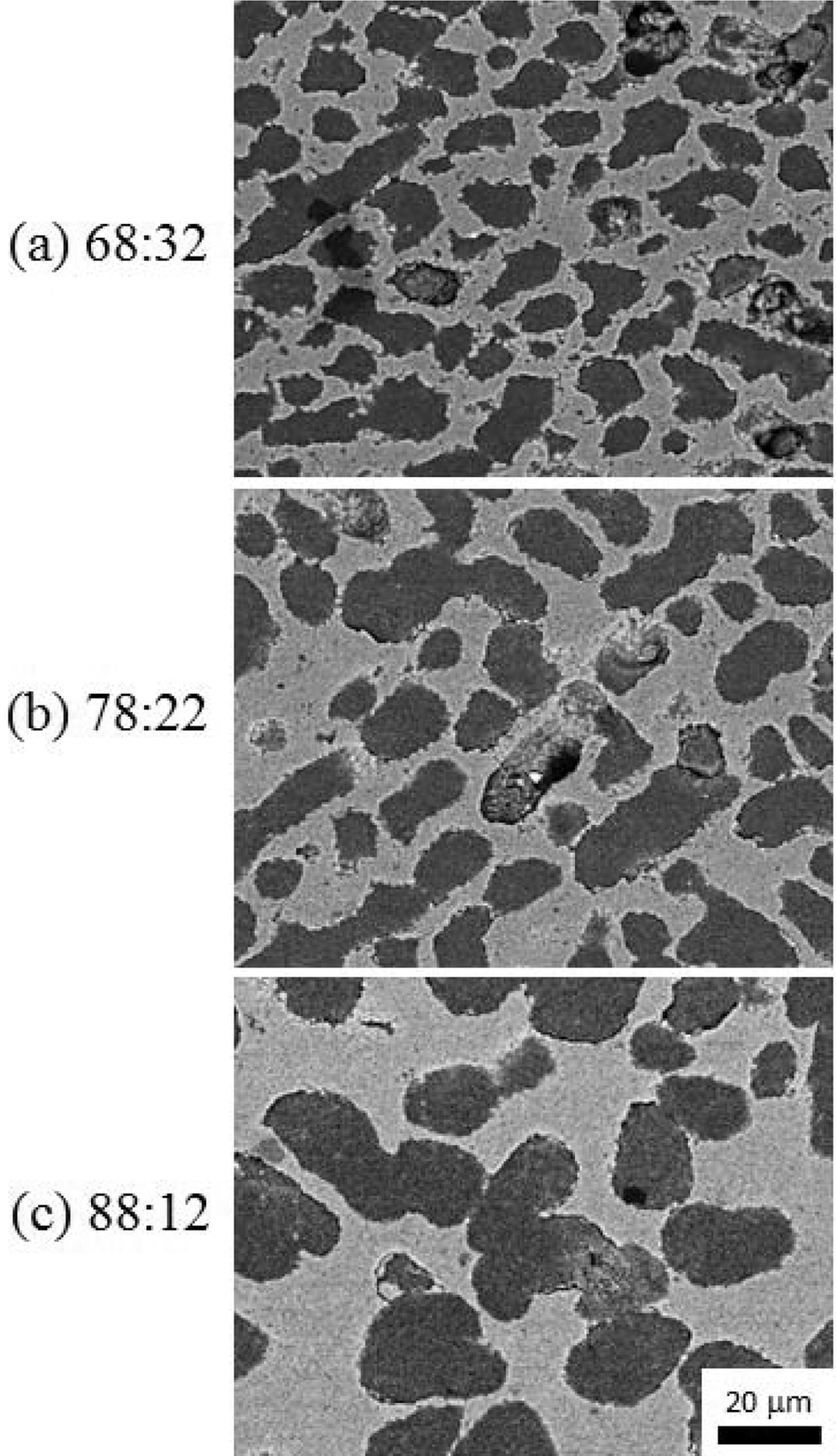
- Fabrication of Porous Al2O3 Film by Freeze Tape Casting
- Ran-Hee Shin, Jun-Mo Koo, Young-Do Kim, Yoon-Soo Han
- J Korean Powder Metall Inst. 2015;22(6):438-442. Published online December 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.6.438
- 686 View
- 6 Download
-
 Abstract
Abstract
 PDF
PDF Porous thick film of alumina which is fabricated by freeze tape casting using a camphene-camphor-acrylate vehicle. Alumina slurry is mixed above the melting point of the camphene-camphor solvent. Upon cooling, the camphene-camphor crystallizes from the solution as particle-free dendrites, with the Al2O3 powder and acrylate liquid in the interdendritic spaces. Subsequently, the acrylate liquid is solidified by photopolymerization to offer mechanical properties for handling. The microstructure of the porous alumina film is characterized for systems with different cooling rate around the melting temperature of camphor-camphene. The structure of the dendritic porosity is compared as a function of ratio of camphene-camphor solvent and acrylate content, and Al2O3 powder volume fraction in acrylate in terms of the dendrite arm width.
TOP
 KPMI
KPMI


 First
First Prev
Prev


