Search
- Page Path
- HOME > Search
- [Korean]
- Effect of Tert-Butyl Alcohol Template on the Pore Structure of Porous Tungsten in Freeze Drying Process
- Eui Seon Lee, Youn Ji Heo, Yun Taek Ko, Jin Gyeong Park, Yong-Ho Choa, Sung-Tag Oh
- J Korean Powder Metall Inst. 2021;28(3):216-220. Published online June 1, 2021
- DOI: https://doi.org/10.4150/KPMI.2021.28.3.216

- 668 View
- 4 Download
-
 Abstract
Abstract
 PDF
PDF The effect of tert-butyl alcohol (TBA) as a freezing solvent on the pore structure of a porous tungsten body prepared by freeze-drying is analyzed. TBA slurries with a WO3 content of 10 vol% are prepared by mixing with a small amount of dispersant and binder at 30°C. The slurries are frozen at -25°C, and pores are formed in the frozen specimens by the sublimation of TBA during drying in air. After hydrogen reduction at 800°C and sintering at 1000°C, the green body of WO3 is completely converted to porous W with various pore structures. Directional pores from the center of the specimen to the outside are observed in the sintered bodies because of the columnar growth of TBA. A decrease in pore directionality and porosity is observed in the specimens prepared by long-duration drying and sintering. The change in pore structure is explained by the growth of the freezing solvent and densification.
- [Korean]
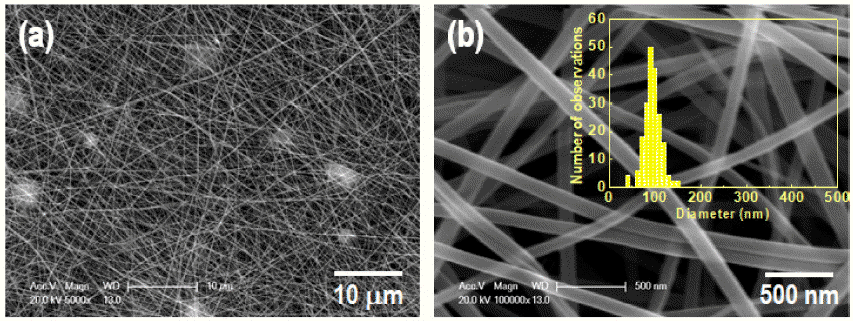
- Effect of Calcination Temperature on the Microstructure and Photocatalytic Activity of Electrospun BiVO4 Nanofiber
- Myeongjun Ji, Jeong Hyun Kim, Cheol-Hui Ryu, Yun Taek Ko, Young-In Lee
- J Korean Powder Metall Inst. 2020;27(3):226-232. Published online June 1, 2020
- DOI: https://doi.org/10.4150/KPMI.2020.27.3.226
- 1,182 View
- 5 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
PDF Bismuth vanadate (BiVO4) is considered a potentially attractive candidate for the visible-light-driven photodegradation of organic pollutants. In an effort to enhance their photocatalytic activities, BiVO4 nanofibers with controlled microstructures, grain sizes, and crystallinities are successfully prepared by electrospinning followed by a precisely controlled heat treatment. The structural features, morphologies, and photo-absorption performances of the asprepared samples are systematically investigated and can be readily controlled by varying the calcination temperature. From the physicochemical analysis results of the synthesized nanofiber, it is found that the nanofiber calcines at a lower temperature, shows a smaller crystallite size, and lower crystallinity. The photocatalytic degradation of rhodamine-B (RhB) reveals that the photocatalytic activity of the BiVO4 nanofibers can be improved by a thermal treatment at a relatively low temperature because of the optimization of the conflicting characteristics, crystallinity, crystallite size, and microstructure. The photocatalytic activity of the nanofiber calcined at 350°C for the degradation of RhB under visible-light irradiation exhibits a greater photocatalytic activity than the nanofibers synthesized at 400°C and 450°C.
-
Citations
Citations to this article as recorded by- Design, synthesis, and characterization of a porous ceramic-supported CeO2 nanocatalyst for CO -free H2 evolution
Jimin Lee, Minseob Lim, Tae Sung Kim, Kee-Ryung Park, Jong-Sik Lee, Hong-Baek Cho, Joo Hyun Park, Yong-Ho Choa
Applied Surface Science.2021; 548: 149198. CrossRef
- Design, synthesis, and characterization of a porous ceramic-supported CeO2 nanocatalyst for CO -free H2 evolution
TOP
 KPMI
KPMI


 First
First Prev
Prev


