Search
- Page Path
- HOME > Search
- [English]
- A Separator with Activated Carbon Powder Layer to Enhance the Performance of Lithium-Sulfur Batteries
- Duc-Luong Vu, Jae-Won Lee
- J Korean Powder Metall Inst. 2018;25(6):466-474. Published online December 1, 2018
- DOI: https://doi.org/10.4150/KPMI.2018.25.6.466
- 1,123 View
- 4 Download
- 2 Citations
-
 Abstract
Abstract
 PDF
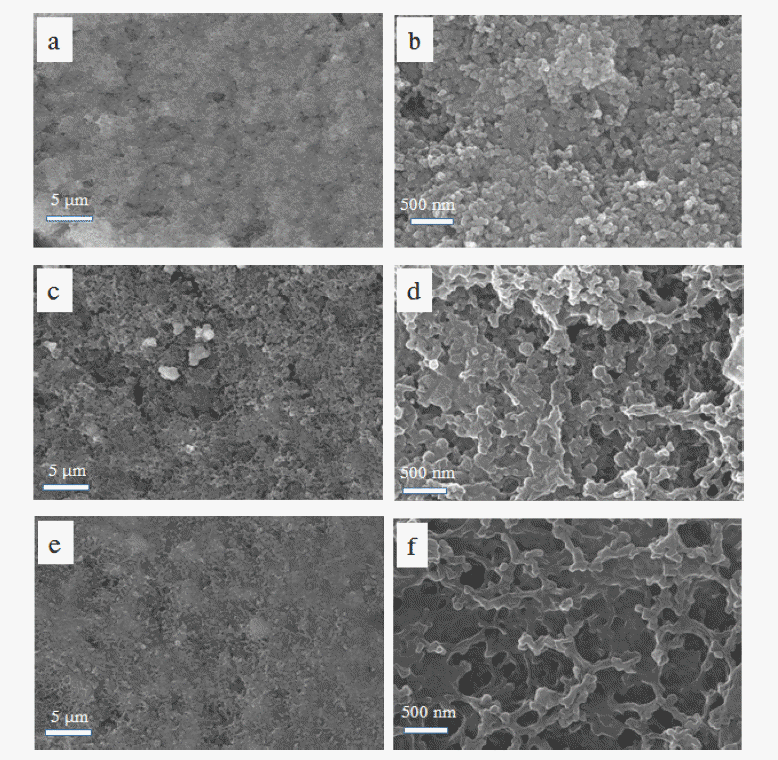
PDF The high theoretical energy density (2600 Wh kg−1) of Lithium-sulfur batteries and the high theoretical capacity of elemental sulfur (1672 mAh g−1) attract significant research attention. However, the poor electrical conductivity of sulfur and the polysulfide shuttle effect are chronic problems resulting in low sulfur utilization and poor cycling stability. In this study, we address these problems by coating a polyethylene separator with a layer of activated carbon powder. A lithium-sulfur cell containing the activated carbon powder-coated separator exhibits an initial specific discharge capacity of 1400 mAh g−1 at 0.1 C, and retains 63% of the initial capacity after 100 cycles at 0.2 C, whereas the equivalent cell with a bare separator exhibits a 1200 mAh g−1 initial specific discharge capacity, and 50% capacity retention under the same conditions. The activated carbon powder-coated separator also enhances the rate capability. These results indicate that the microstructure of the activated carbon powder layer provides space for the sulfur redox reaction and facilitates fast electron transport. Concurrently, the activated carbon powder layer traps and reutilizes any polysulfides dissolved in the electrolyte. The approach presented here provides insights for overcoming the problems associated with lithium-sulfur batteries and promoting their practical use.
-
Citations
Citations to this article as recorded by- A one-step deposition method to prepare separators with carbon soot loading for lithium-sulfur battery
Yueting Zhu, Jingjing Wang, Yanshu Wang, Ying Zhu, Yixuan Li, Shicheng Zhao
Ionics.2022; 28(4): 1693. CrossRef - High thermal stability multilayered electrolyte complexes via layer-by-layer for long-life lithium-sulfur battery
Jing Wang, Yufan Li, Xianmei Deng, Lei Yan, Zhiqiang Shi
Ionics.2020; 26(11): 5481. CrossRef
- A one-step deposition method to prepare separators with carbon soot loading for lithium-sulfur battery
TOP
 KPMI
KPMI


 First
First Prev
Prev


