Search
- Page Path
- HOME > Search
- [Korean]
- Recovery of Tungsten from WC/Co Hardmetal Sludge by Alkaline Leaching Hydrometallurgy Process
- Gil-Geun Lee, Ji-Eun Kwon
- J Korean Powder Metall Inst. 2016;23(5):372-378. Published online October 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.5.372
- 839 View
- 3 Download
- 1 Citations
-
 Abstract
Abstract
 PDF
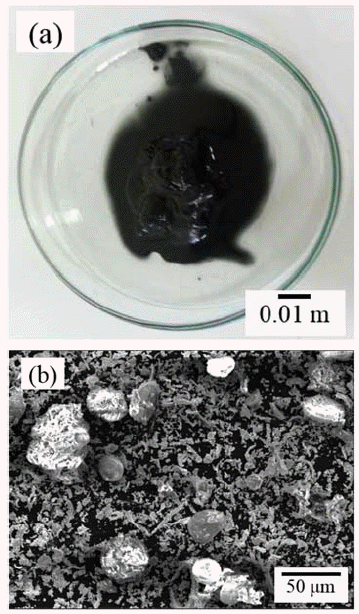
PDF This study focuses on the development of an alkaline leaching hydrometallurgy process for the recovery of tungsten from WC/Co hardmetal sludge, and an examination of the effect of the process parameters on tungsten recovery. The alkaline leaching hydrometallurgy process has four stages, i.e., oxidation of the sludge, leaching of tungsten by NaOH, refinement of the leaching solution, and precipitation of tungsten. The WC/Co hardmetal sludge oxide consists of WO3 and CoWO4. The leaching of tungsten is most affected by the leaching temperature, followed by the NaOH concentration and the leaching time. About 99% of tungsten in the WC/Co hardmetal sludge is leached at temperatures above 90°C and a NaOH concentration above 15%. For refinement of the leaching solution, pH control of the solution using HCl is more effective than the addition of Na2S·9H2O. The tungsten is precipitated as high-purity H2WO4·H2O by pH control using HCl. With decreasing pH of the solution, the tungsten recovery rate increases and then decrease. About 93% of tungsten in the WC/Co hardmetal sludge is recovered by the alkaline leaching hydrometallurgy process.
-
Citations
Citations to this article as recorded by- Fabrication of tungsten oxide powder from WC–Co cemented carbide scraps by oxidation behaviour
Min Soo Park, Jong-Min Gwak, Kyeong-mi Jang, Gook-Hyun Ha
Powder Metallurgy.2023; 66(5): 688. CrossRef
- Fabrication of tungsten oxide powder from WC–Co cemented carbide scraps by oxidation behaviour
- [Korean]
- Briquetting of Waste Silicon Carbide Obtained from Silicon Wafer Sludges
- Seong Mo Koo, Su Jong Yoon, Hye Sung Kim
- J Korean Powder Metall Inst. 2016;23(1):43-48. Published online February 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.1.43
- 767 View
- 6 Download
-
 Abstract
Abstract
 PDF
PDF Waste SiC powders obtained from silicon wafer sludge have very low density and a narrow particle size distribution of 10-20 μm. A scarce yield of C and Si is expected when SiC powders are incorporated into the Fe melt without briquetting. Here, the briquetting variables of the SiC powders are studied as a function of the sintering temperature, pressure, and type and contents of the binders to improve the yield. It is experimentally confirmed that Si and C from the sintered briquette can be incorporated effectively into the Fe melt when the waste SiC powders milled for 30 min with 20 wt.% Fe binder are sintered at 1100°C upon compaction using a pressure of 250 MPa. XRF-WDS analysis shows that an yield of about 90% is obtained when the SiC briquette is kept in the Fe melt at 1650°C for more than 1 h.
TOP
 KPMI
KPMI


 First
First Prev
Prev


