Search
- Page Path
- HOME > Search
- [English]
- Fabrication and Characterization of Thermal Battery using Porous MgO Separator Infiltrated with Li based Molten Salts
- Kyungho Kim, Sungmin Lee, Chae-Nam Im, Seung-Ho Kang, Hae-Won Cheong, Yoonsoo Han
- J Korean Powder Metall Inst. 2017;24(5):364-369. Published online October 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.5.364
- 617 View
- 5 Download
-
 Abstract
Abstract
 PDF
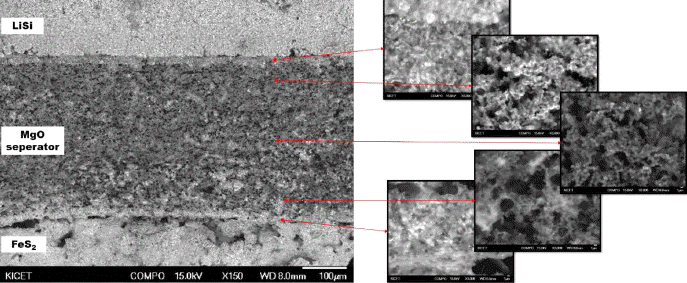
PDF Ceramic powder, such as MgO, is added as a binder to prepare the green compacts of molten salts of an electrolyte for a thermal battery. Despite the addition of a binder, when the thickness of the electrolyte decreases to improve the battery performance, the problem with the unintentional short circuit between the anode and cathode still remains. To improve the current powder molding method, a new type of electrolyte separator with porous MgO preforms is prepared and characteristics of the thermal battery are evaluated. A Spherical PMMA polymer powder is added as a pore-forming agent in the MgO powder, and an organic binder is used to prepare slurry appropriate for tape casting. A porous MgO preform with 300 μm thickness is prepared through a binder burnout and sintering process. The particle size of the starting MgO powder has an effect, not on the porosity of the porous MgO preform, but on the battery characteristics. The porosity of the porous MgO preforms is controlled from 60 to 75% using a pore-forming agent. The batteries prepared using various porosities of preforms show a performance equal to or higher than that of the pellet-shaped battery prepared by the conventional powder molding method.
- [Korean]
- Fabrication of Solid State Electrolyte Li7La3Zr2O12 thick Film by Tape Casting
- Ran-Hee Shin, Samick Son, Sung-Soo Ryu, Hyung-Tae Kim, Yoon-Soo Han
- J Korean Powder Metall Inst. 2016;23(5):379-383. Published online October 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.5.379
- 775 View
- 10 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
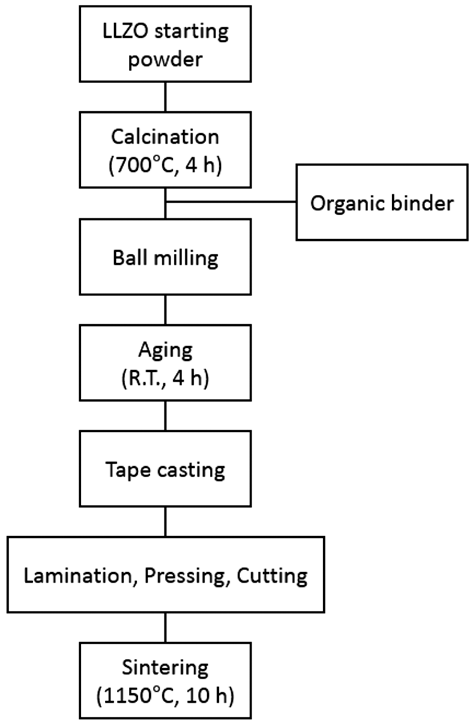
PDF A thick film of Li7La3Zr2O12 (LLZO) solid-state electrolyte is fabricated using the tape casting process and is compared to a bulk specimen in terms of the density, microstructure, and ion conductivity. The final thickness of LLZO film after sintering is 240 μm which is stacked up with four sheets of LLZO green films including polymeric binders. The relative density of the LLZO film is 83%, which is almost the same as that of the bulk specimen. The ion conductivity of a LLZO thick film is 2.81 × 10−4 S/cm, which is also similar to that of the bulk specimen, 2.54 × 10−4 S/ cm. However, the microstructure shows a large difference in the grain size between the thick film and the bulk specimen. Although the grain boundary area is different between the thick film and the bulk specimen, the fact that both the ion conductivities are very similar means that no secondary phase exists at the grain boundary, which is thought to originate from nonstoichiometry or contamination.
-
Citations
Citations to this article as recorded by- Waste minimization in all-solid-state battery production via re-lithiation of the garnet solid electrolyte LLZO
Vivien Kiyek, Martin Hilger, Melanie Rosen, Jürgen Peter Gross, Markus Mann, Dina Fattakhova-Rohlfing, Ruth Schwaiger, Martin Finsterbusch, Olivier Guillon
Journal of Power Sources.2024; 609: 234709. CrossRef - Powder Aerosol Deposition as a Method to Produce Garnet‐Type Solid Ceramic Electrolytes: A Study on Electrochemical Film Properties and Industrial Applications
Tobias Nazarenus, Yanyan Sun, Jörg Exner, Jaroslaw Kita, Ralf Moos
Energy Technology.2021;[Epub] CrossRef - Synthesize of Nd2Fe14B Powders from 1-D Nd2Fe14B Wires using Electrospinning Process
Nu Si A Eom, Su Noh, Muhammad Aneeq Haq, Bum Sung Kim
Journal of Korean Powder Metallurgy Institute.2019; 26(6): 477. CrossRef
- Waste minimization in all-solid-state battery production via re-lithiation of the garnet solid electrolyte LLZO
- [Korean]
- Fabrication of Porous Al2O3 Film by Freeze Tape Casting
- Ran-Hee Shin, Jun-Mo Koo, Young-Do Kim, Yoon-Soo Han
- J Korean Powder Metall Inst. 2015;22(6):438-442. Published online December 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.6.438
- 513 View
- 5 Download
-
 Abstract
Abstract
 PDF
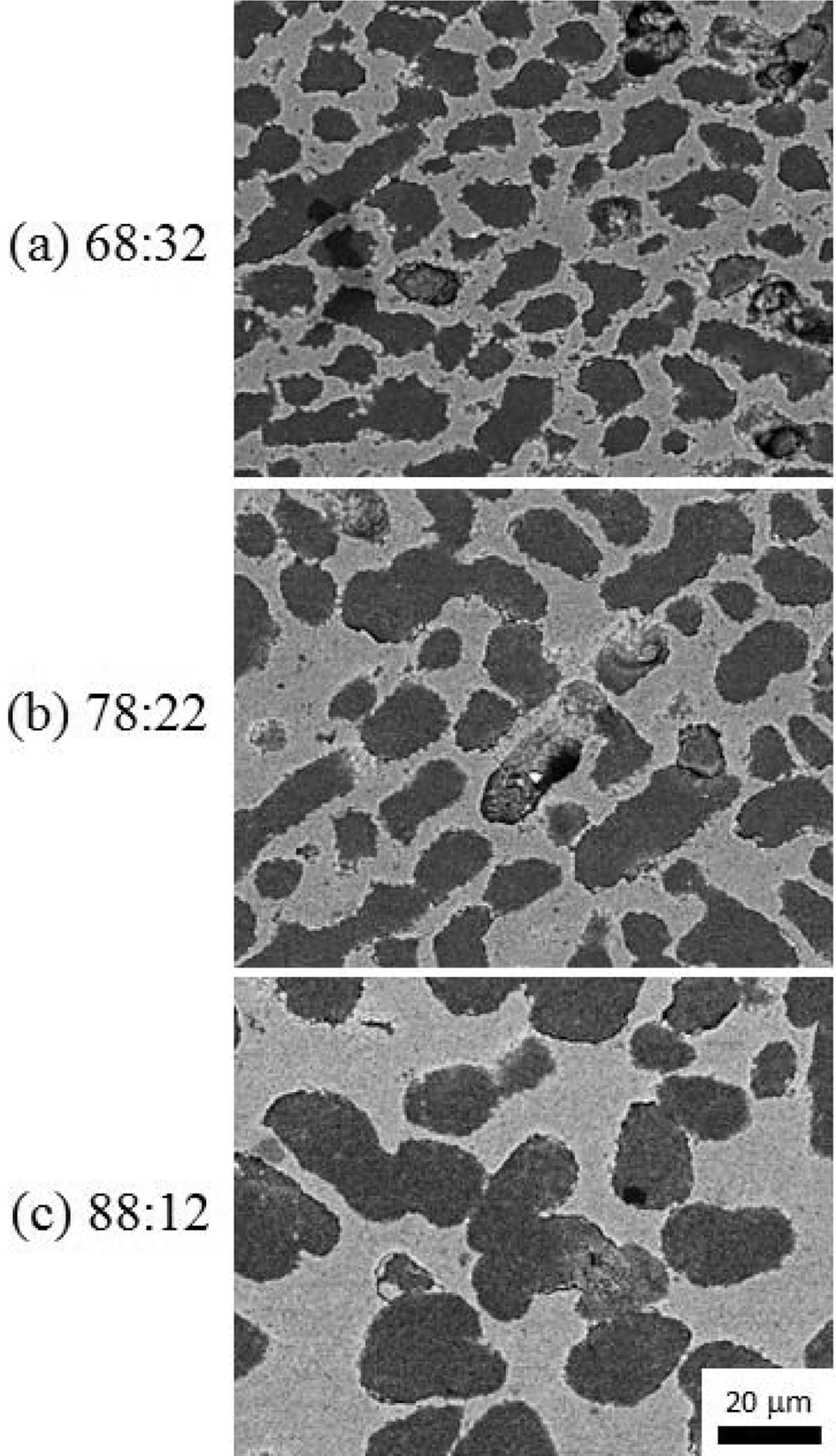
PDF Porous thick film of alumina which is fabricated by freeze tape casting using a camphene-camphor-acrylate vehicle. Alumina slurry is mixed above the melting point of the camphene-camphor solvent. Upon cooling, the camphene-camphor crystallizes from the solution as particle-free dendrites, with the Al2O3 powder and acrylate liquid in the interdendritic spaces. Subsequently, the acrylate liquid is solidified by photopolymerization to offer mechanical properties for handling. The microstructure of the porous alumina film is characterized for systems with different cooling rate around the melting temperature of camphor-camphene. The structure of the dendritic porosity is compared as a function of ratio of camphene-camphor solvent and acrylate content, and Al2O3 powder volume fraction in acrylate in terms of the dendrite arm width.
TOP
 KPMI
KPMI


 First
First Prev
Prev


