Articles
- Page Path
- HOME > J Korean Powder Metall Inst > Volume 22(5); 2015 > Article
-
ARTICLE
- Synthesis and Characterization of Tungsten Trioxide Films Prepared by a Sol-Gel Method for Electrochromic Applications
- Tae-Ho Kim, Yoon-Chae Nah*
-
Journal of Korean Powder Metallurgy Institute 2015;22(5):309-314.
DOI: https://doi.org/10.4150/KPMI.2015.22.5.309
Published online: September 30, 2015
Interdisciplinary Program in Creative Engineering, School of Energy, Materials, and Chemical Engineering,Korea University of Technology and Education, Cheonan 31253, Korea
- *Corresponding author : : Yoon-Chae Nah, TEL: +82-41-560-1322, FAX: +82-41-560-1370,ycnah@koreatech.ac.kr
• Received: October 1, 2015 • Revised: October 20, 2015 • Accepted: October 19, 2015
© The Korean Powder Metallurgy Institute All rights reserved
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 753 Views
- 5 Download
- 1 Crossref
Abstract
- Tungsten trioxide thin films are successfully synthesized by a sol-gel method using tungsten hexachloride as precursors. The structural, chemical, and optical properties of the prepared films are characterized by scanning electron microscopy, X-ray diffraction, X-ray photoelectron spectroscopy, and UV-Vis spectrophotometry. The electrochemical and electrochromic properties of the films before and after heat treatment are also investigated by cyclic voltammetry, chronoamperometry, and in situ transmittance measurement system. Compared to as-prepared films, heattreated tungsten trioxide thin films exhibit a higher electrochemical reversibility of 0.81 and superior coloration efficiency of 65.7 cm2/C, which implies that heat treatment at an appropriate temperature is a crucial process in a sol-gel method for having a better electrochromic performance.
- Electrochromism refers to the reversible changes in optical properties of materials when they undergo oxidation or reduction reactions under applied voltages [1,2]. Several electrochromic materials, including transition metal oxides, organic molecules, and conjugated polymers, have been reported extensively [3-5]. Transition metal oxides can be used in smart glass systems [2,6] and in the rear-view mirrors of automobiles [7] due to their high durability and chemical stability. However, organic molecules and polymers exhibit various colorations with a higher response speed, allowing them to be applied to informational display devices [8,9].
- Among these electrochromic materials, tungsten trioxide has been widely studied due to its high electrochromic efficiency, large dynamic range, and cyclic reversibility [3,10]. Tungsten trioxide is a cathodic coloration material that possesses a coloured state in its reduced formed [2,11]. This material has an energy band gap of about 3.0 eV [12] and an almost transparent appearance because of its inability to absorb visible light [13]. Following its reduction, tungsten trioxide has a strong absorption near 600 nm and a blue colour because of the formation of tungsten bronze (MxWO3) via the following reaction.
- WO3 + xe- + xM+ ↔ MxWO3 (M: H, Li, Na, etc.)
- A variety of methods can be used to prepare tungsten trioxide films, such as vacuum evaporation [14], sputtering [15], chemical vapor deposition [16], electrodeposition [17], and sol-gel processes [18]. Sol-gel processes offers several advantages over conventional deposition techniques for preparing tungsten trioxide films by enabling precise control of stoichiometry and morphology, which, in turn, can influence the kinetics, reversibility, and coloration efficiency of electrochromic films [19].
- The present study describes the development of tungsten trioxide films deposited by a sol–gel method using tungsten hexachloride as precursors on conductive glass substrates. The electrochemical properties, coloration efficiencies, and electrochromic responses of the films before and after heat treatment are also determined in this work.
Introduction
- Indium tin oxide (ITO, 8-10 Ω/square) coated glass was used as transparent conductive electrodes. The ITO glass was cleaned before use by sonication in isopropyl alcohol, acetone, ethanol, and deionized (DI) water, successively, for 5 min. The substrate was then dried with nitrogen. Tungsten hexachloride (WCl6) (Aldrich Chem, 99.99%) was used as the precursor for coating tungsten trioxide. A 0.5 M WCl6 solution was prepared in 3 ml ethanol (Aldrich Chem., 99.9%) with 10 wt% glycerol as a binder material. The solution was stirred for 12 h until it turned green. The solution was spin-coated on the ITO substrate at 2000 rpm for 20 s. The coated films were dried in air at room temperature and then annealed at 190°C for 2.5 h.
- The morphology and microstructure of the coated films were examined by field emission scanning electron microscopy (SEM, Hitachi S-4700) and X-ray diffraction (XRD, Rigaku Rint 2500, Cu Kα source (λ = 1.54Å), 40 kV, 40 mA). To determine the composition and chemical states of the samples, energy dispersive X-ray spectroscopy (EDX) and X-ray photoelectron spectroscopy (XPS, ESCLAB 250, X-ray source Al Kα) analysis were performed.
- All electrochemical tests were performed with a three electrode cell at room temperature. Tungsten trioxide film on the ITO glass substrate was used as the working electrode. Pt and Ag wires were used as the counter and reference electrodes, respectively. A 0.5 M LiClO4 solution in propylene carbonate was used as the electrolyte.
- Electrochemical experiments were performed with an Autolab PGSTAT30 potentiostat/galvanostat. Cyclic voltammetry tests were performed in the potential range between -0.6 V and 1.0 V at a scan rate of 50 mV/s The electrochromic coloration and response time were tested by applying pulse potentials between -0.6 V and 1.0 V with an interval time of 30 s. Optical transmittance was monitored by means of an in situ transmittance system, using a UV-Vis spectrophotometer (HP 8453) during all experiments. The transmittance of the ITO coated glass, surrounding electrolyte, and cell window was assumed to be 100%.
Experimental
- Fig. 1 shows SEM images and EDX data of spincoated tungsten trioxide films prepared by the sol-gel method described previously. As shown in Fig. 1(a), a fresh film has a rough surface morphology with microcracks, indicating that as-prepared tungsten trioxide films do not form dense and uniform structures. Moreover, it was observed in the EDX spectra shown in the inset to Fig. 1(a) that some amount of chlorine existed within the films. However, after heat treatment at 190°C for 2.5 h, a tungsten trioxide film with a dense and uniform surface morphology and without defects or elemental chlorine was achieved as shown in Fig 1(b). These findings suggest that a heat treatment process is necessary after the sol-gel coating to obtain a film structure without defects and to eliminate film impurities.
- In order to investigate the microstructure of heattreated tungsten trioxide films, XRD measurement was conducted. Fig. 2(a) shows the XRD patterns of the silicon substrate and tungsten trioxide films coated on the silicon substrate. Due to offset angle used in XRD measurement, the diffraction peaks at 28.4°, 47.3°, and 56.1° indexed respectively to the (111), (220), and (311) planes of Si crystallites do not appear. Compared to the pure silicon substrate, tungsten trioxide/silicon displayed broad peaks near 2θ = 23.2°, 23.6°, and 24.4°, which were assigned to the (001), (020), and (200) planes of monoclinically structured tungsten trioxide [20], respectively. Other diffraction peaks at 33.3°, 47.0°, and 50.4° were also attributed to the monoclinic structure of tungsten trioxide. However, the peak intensities were relatively weak with a broad background, indicating a considerable amount of amorphous material was present. tungsten trioxide has been reported to have a clear monoclinic phase after heat treatment above 450°C [3].
- The chemical states of tungsten trioxide films fabricated by the sol-gel method were measured using XPS. The XPS spectra collected for tungsten trioxide confirms the presence of W and O as well as a small amount of C, while no signal from impurities was observed in the sample. Carbon is believed to be introduced during sample preparation and handling. The high resolution W4f spectrum (Fig. 2(b)) has the characteristic W 4f5/2 and W 4f7/2 peaks for tungsten trioxide. The binding energies of W4f5/2 and W 4f7/2 were centered at 38.0 eV and 35.8 eV, respectively. According to previous reports, the binding energy of W6+ is 35.8 eV [21], indicating that the tungsten trioxide films prepared by the sol-gel method were in good agreement with the chemical ratio of WO3.
- Fig. 2(c) shows the optical transmittance and absorption spectra of tungsten trioxide films, which were prepared by spin-coating followed by heat treatment at 190 °C. The film was highly transparent with a pale yellow color and transmittance of over 80% in the visible light region. In accordance with previous studies that found the energy band gap of crystalline tungsten trioxide to be approximately 3.0 eV [12], the films prepared using the sol-gel method had a very similar energy band gap. Additionally, the high transmittance of these tungsten trioxide films makes them highly promising materials for applications in smart windows.
- Cyclic voltammetry was performed in order to study the electrochemical properties of the spin-coated tungsten trioxide films. As shown in Fig. 3(a), the voltametric curves had the typical form expected for amorphous tungsten trioxide. However, a small cathodic peak was observed at -0.2 V, reflecting the crystalline nature of the tungsten trioxide film [1]. Therefore, a mixture of amorphous and crystalline phases was confirmed by the electrochemical test in agreement with the XRD results. The curve has a maximum reduction current density of 0.2 mA/cm2 at -0.6 V and a maximum oxidation current density of 0.15 mA/cm2 at -0.4 V. During the cathodic reaction, an intercalation of Li+ ions from the electrolyte with the tungsten trioxide lattice occurred, forming deep blue LixWO3. A reversible anodic reaction led to the recovery of tungsten trioxide by deintercalation of Li+ ions, and the sample became transparent (see the inset of Fig. 3(a)). For as prepared tungsten trioxide films, the small peak at -0.3 V was also observed, but there is not distinguishable peak (Fig. 3(b)). Moreover, current density of non heat-treated sample is small. This is possibly due to the fact that the impurities such as chlorine of tungsten trioxide films can cause the reduction of charge transfer rates, which affects diffusion dynamics during the redox reactions. Diffusion coefficients for two samples were estimated by using Randles-Servcik equation below.
- Where ip is the peak current density, D is diffusion coefficient, C0 indicates the concentration of active ions in the electrolyte, and v is scan rate during the redox reactions (n is assumed to be 1 [22].)
- The diffusion coefficients of tungsten trioxide films before and after heat treatment were 1.32 × 10–11 cm2/s, and 1.55 × 10–11 cm2/s which is calculated using anodic peak currents, respectively. Based on cathodic peak currents, the diffusion coefficients were 3.97 × 10–11 cm2/s and 4.74 × 10– 11 cm2/s. These results indicate that removal of chlorine after the heat treatment is necessary for better ion diffusion rates.
- Fig. 4 shows the results of chronoamperometry at -0.6 V and 1.0 V for 30 s. After heat treatments, the tungsten trioxide films shows highly enhanced performance; the cathodic and anodic charge densities after heat treatment were 5.53 mC/cm2 (Qc) and 4.46 mC/cm2 (Qa) during reduction and oxidization, respectively. However, for tungsten trioxide films before heat treatment, it was 1.91 mC/cm2 (Qc) and 1.18 mC/cm2 (Qa). In addition, the ratio Qa/Qc of the films before and after heat treatment is 62% and 81%, respectively, which means that electrochemical reaction was highly enhanced after heat treatment.
- The electrochromic properties were monitored via in situ transmittance test shown in Fig. 5. For heat treated sample (Fig. 5(a)), the transmittance was 35.3% at a colored state and 91.8% at a bleached state. The difference in transmittance was 56.5%. The response time was estimated from time required for 90% of the total transmittance difference to be reached. Response time of 6.7 s and 2.6 s was observed for coloring and bleaching processes, respectively. However, tungsten trioxide films before heat treatment in Fig. 5(b) shows insufficient transmittance difference of 18.4% with response time of 7.1 s and 1.2 s during coloring and bleaching process, respectively. Overall, these findings imply that electrochromic performance is also notably affected by the addition of heat treatment. Combined with the results of Fig. 4 and 5, the coloration efficiency (CE) was calculated on the basis of the following equation [23,24].
- The CE of the tungsten trioxide films prepared with heat treatment was approximately 65.7 cm2/C. The CE of tungsten trioxide films after heat treatment is comparable to the values prepared by other process (40-70 cm2/ C), [25] which means heat treatment is very important process for the high electrochromic performance.
Results and Discussion
Fig. 1.

SEM images of the surface of the tungsten trioxide films fabricated by the sol-gel method (a) before and (b) after heat treatment. The insets show EDX results.

Fig. 2.
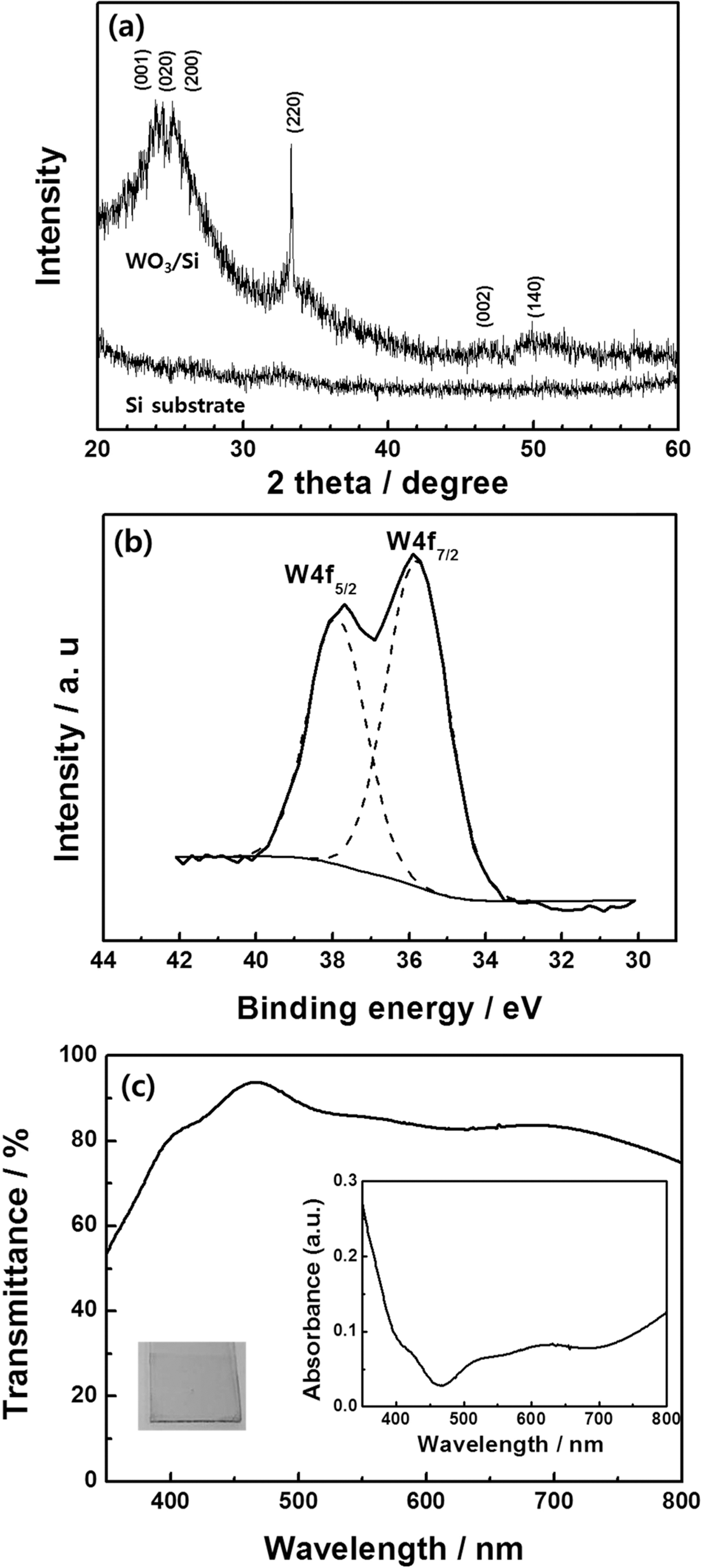
(a) XRD patterns of the silicon substrate and tungsten trioxide/silicon, (b) XPS analysis of tungsten trioxide, and (c) transmittance (absorption in inset) of tungsten trioxide film. The inset is a photograph of a tungsten trioxide film coated on an ITO substrate.

Fig. 3.
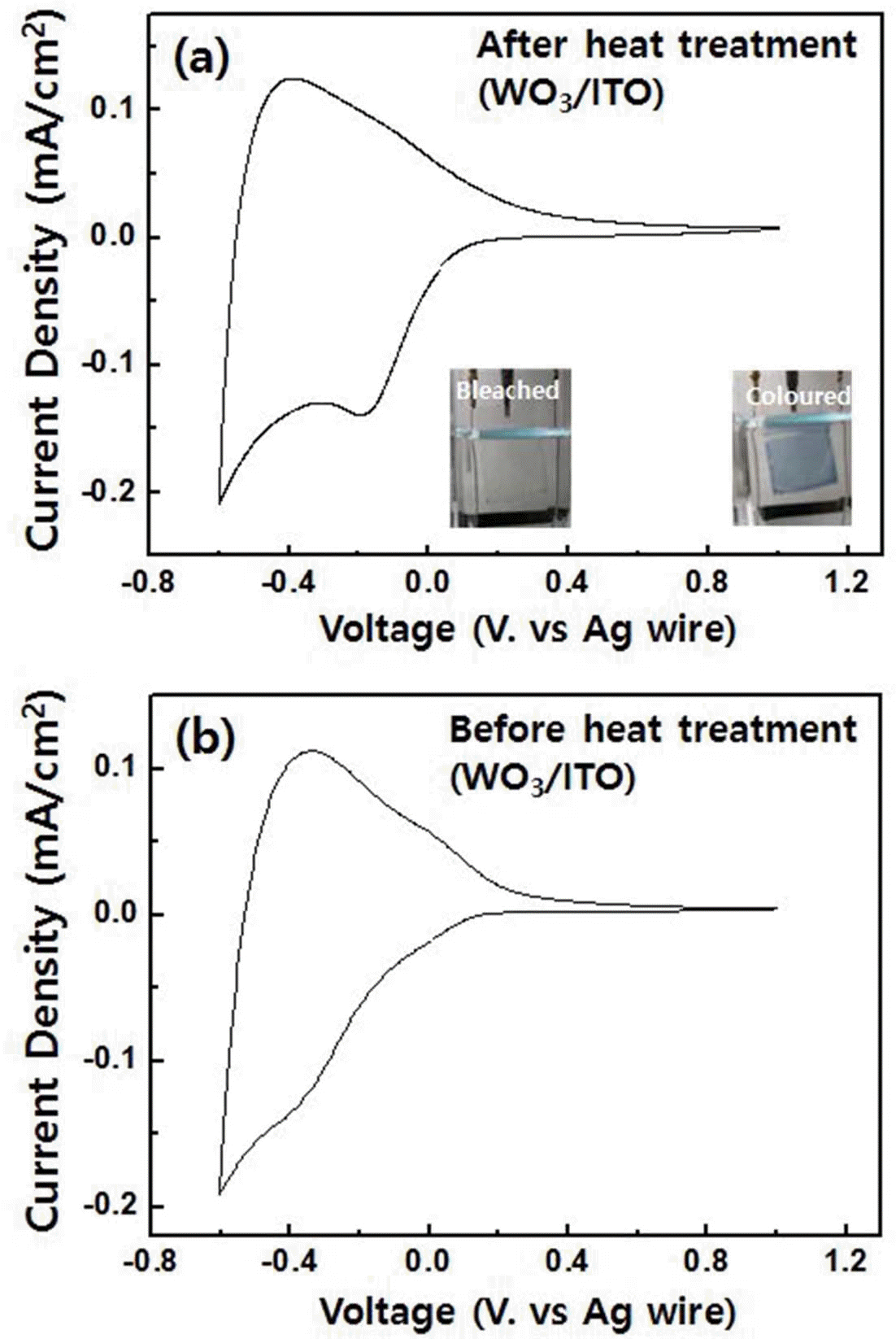
The results of cyclic voltammetry, (a) after and (b) before heat treatment of tungsten trioxide film, performed at -0.6 V and 1.0 V with 50 mV/s scan rate. The colored and bleached states of tungsten trioxide are shown in the inset of Fig. 3(a).

Fig. 4.
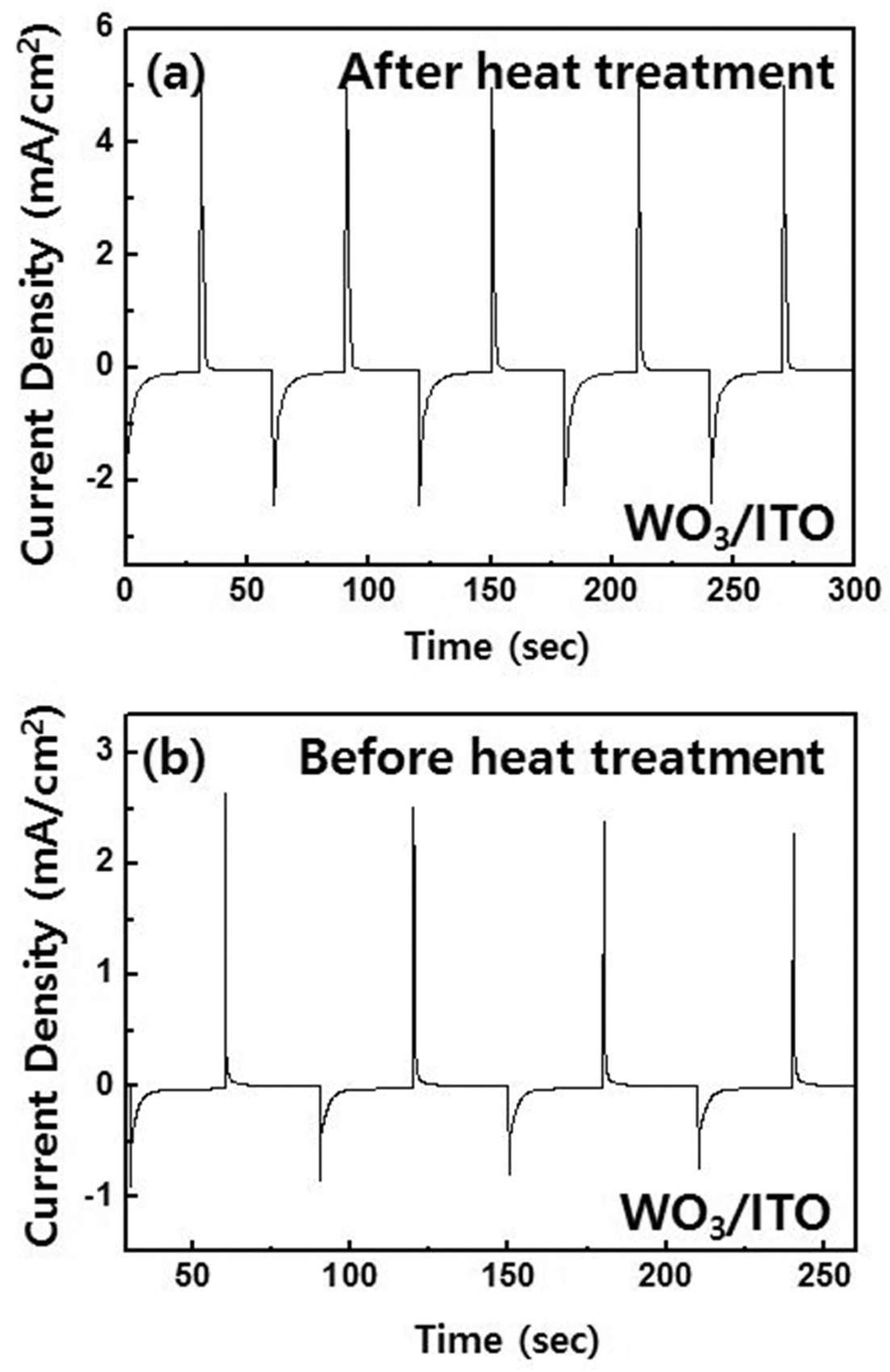
The results of chronoamperometry performed at -0.6 V and 1.0 V for tungsten trioxide film. (a) after and (b) before heat treatment.

Fig. 5.
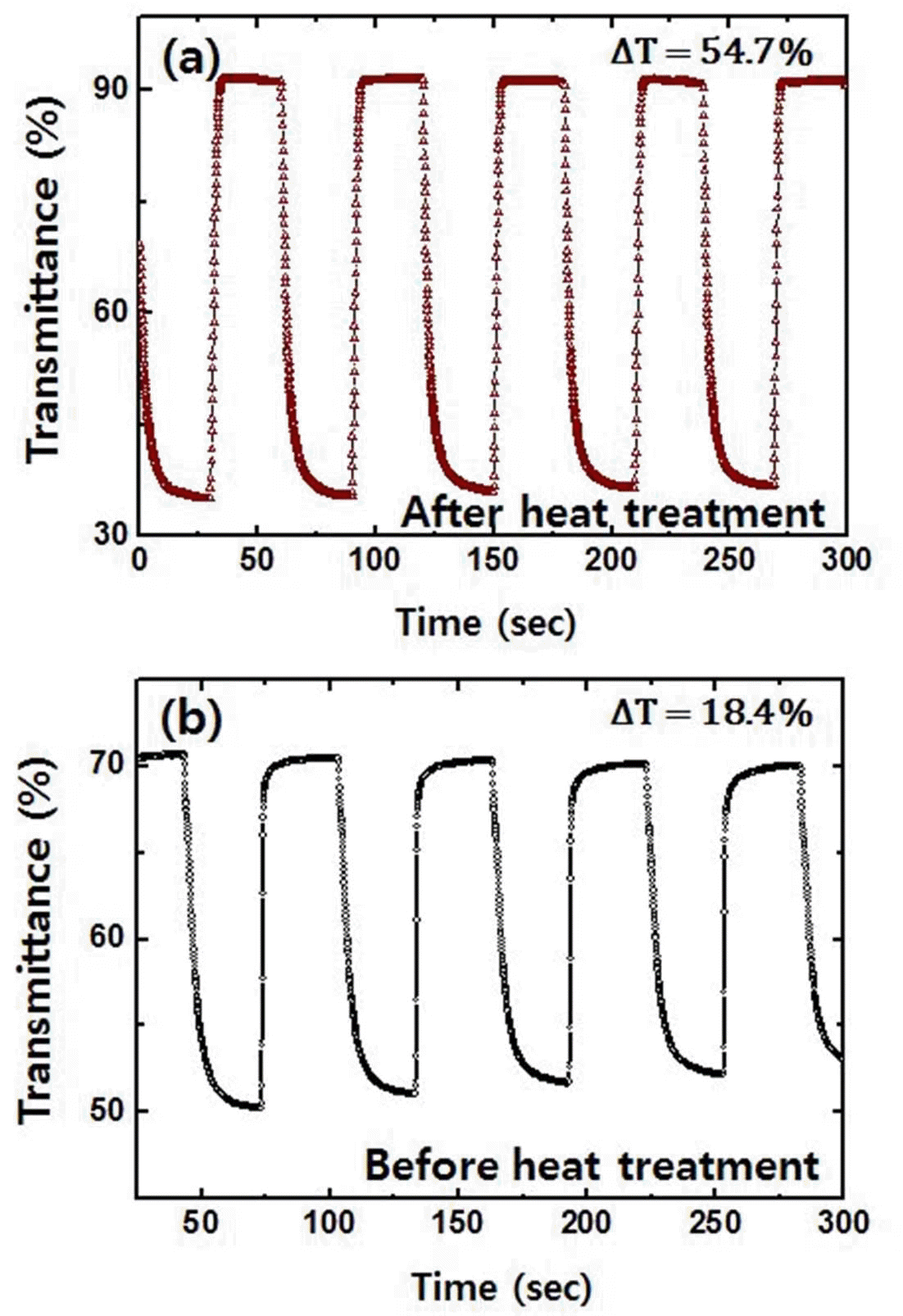
Transmittance data collected during chronoamperometry test performed at -0.6 V and 1.0 V for tungsten trioxide film (a) after and (b) before heat treatment.

- In this study, tungsten trioxide films were successfully fabricated using a sol-gel method. The structural, chemical, electrochemical, and electrochromic properties of these films were investigated. A uniform, dense film was obtained after annealing at 190°C. The structure of the tungsten trioxide films was highly stoichiometric and amorphous with a small monoclinic phase. From the electrochemical and electrochromic analysis, the tungsten trioxide films after heat treatment were confirmed to be very highly reversible with comparable electrochromic efficiencies to similar structures. By comparison with as-prepared tungsten trioxide films, it is concluded that heat treatment at an appropriate temperature is a crucial process in a sol-gel method to improve electrochemical and electrochromic performance.
Conclusions
-
Acknowledgements
- This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2012R1A1A1014075) and also supported by the Research Program of Semiconductor/ Display Cluster in Korea University of Technology and Education.
Acknowledgements
- 1. PMS Monk, RJ Mortimer and D R Rosseinsky, Electrochromism: Fundamentals and Applications. (2007) Wiley-VCH Verlag GmbH, Weinheim 22.
- 2. SK Deb, Sol Energy Mater. Sol. Cells. (2008) 92 –245.
- 3. YC Nah, A Ghicov, D Kim and P Schmuki, Electrochem Commun. (2008) 10 –1777.
- 4. YC Nah, J Nanosci. Nanotechnol. (2013) 13 –3270.
- 5. J Zhu, S Wei, L Zhang, Y Mao, J Ryu, ABD Karki, P Young and Z Guo, J Mater. Chem. (2011) 21 –342.
- 6. A Azens and C Granqvist, J Solid State Electrochem. (2003) 7 –64.
- 7. PMS Monk, RJ Mortimer and DR Rosseinsky, Electrochromism: Fundamentals and Applications. (2007) Wiley. VCH Verlag GmbH, Weinheim 42.
- 8. PR Somani and S Radhakrishnan, Mater Chem. Phys. (2003) 77 –117.
- 9. RJ Mortimer, AL Dyer and JR Reynolds, Displays. (2006) 27 –2.
- 10. M Seman and CA Wolden, Sol Energy Mater. Sol. Cells. (2004) 82 –517.
- 11. TH Kim, HJ Jeon, JW Lee and YC Nah, Elctrochem Commun. (2015) 57 –65.
- 12. V Keller and F Garin, Catal Commun. (2003) 4 –377.
- 13. M Sun, N Xu, YW Cao, JN Yao and EG Wang, J Mater. Sci. Lett. (2000) 19 –1407.
- 14. B Reichman and AJ Bard, J Electrochem. Soc. (1979) 126 –583.
- 15. Z Liu, T Yamazaki, Y Shen, T Kikuta and N Nakatani, Sens Actuators B. (2007) 128 –173.
- 16. T Maruyama and S Arai, J Electrochem. Soc. (1994) 141 –1021.
- 17. L Su, L Zhang, J Fang, M Xu and Z Lu, Sol Energy Mater. Sol. Cells. (1999) 58 –133.
- 18. R Solarska, B Alexander and J Augustynski, J Solid State Electrochem. (2004) 8 –748.
- 19. M Breedon, P Spizzirri, M Taylor, J du Plessis, D McCulloch, J Zhu, L Yu, Z Hu, C Rix, W Wlodarski and K Kalantar-Zadeh, Cryst Growth Des. (2010) 10 –430.
- 20. LE Fraga and MVB Zanoni, J Braz. Chem. Soc. (2011) 22 –718.
- 21. SR Bath and PS Patil, Smart Mater Struct. (2009) 18 –025004.
- 22. R Vijayalakshmi, M Jayachandran and C Sanjeeviraja, Curr Appl. Phys. (2003) 3 –171.
- 23. YC Nah, WS Choi and DY Kim, Sol Energy Mater. Sol. Cells. (2008) 92 –1547.
- 24. L Meda, RC Breitkopf, TE Haas and RU Kirss, Thin Solid Films. (2002) 402 –126.
- 25. CG Granqvist, Electrochim Acta. (1999) 44 –3005.
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Temperature-dependent electrochromic cycling performance of solution-processed WO3 films
Hoon Kang, Gyung Hyun Kim, Sungho Kang, Tae Hoon Park, Kwanchul Kim, Hwan Kyu Kim, Yekyung Kim
Nanoscale.2025; 17(35): 20135. CrossRef
Synthesis and Characterization of Tungsten Trioxide Films Prepared by a Sol-Gel Method for Electrochromic Applications

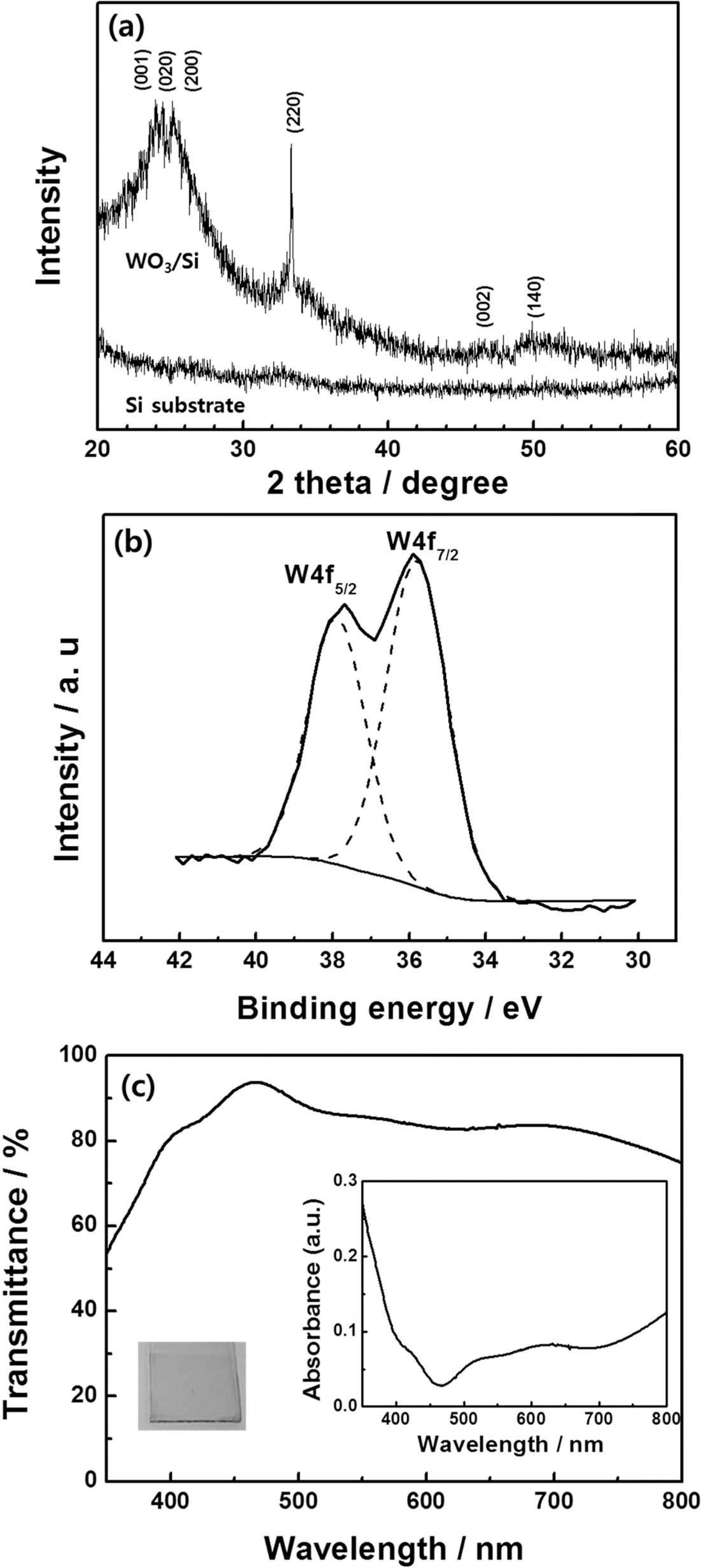
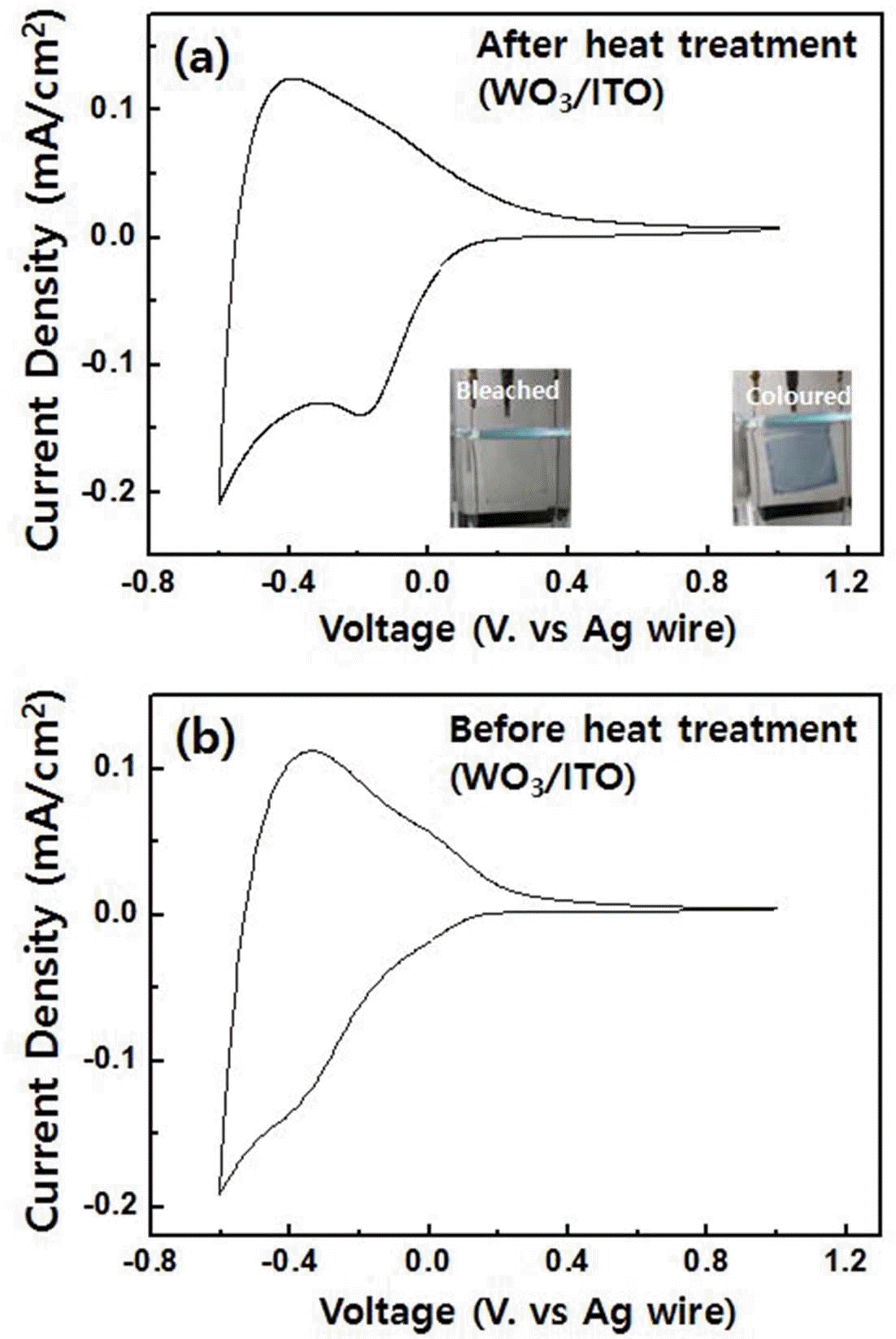
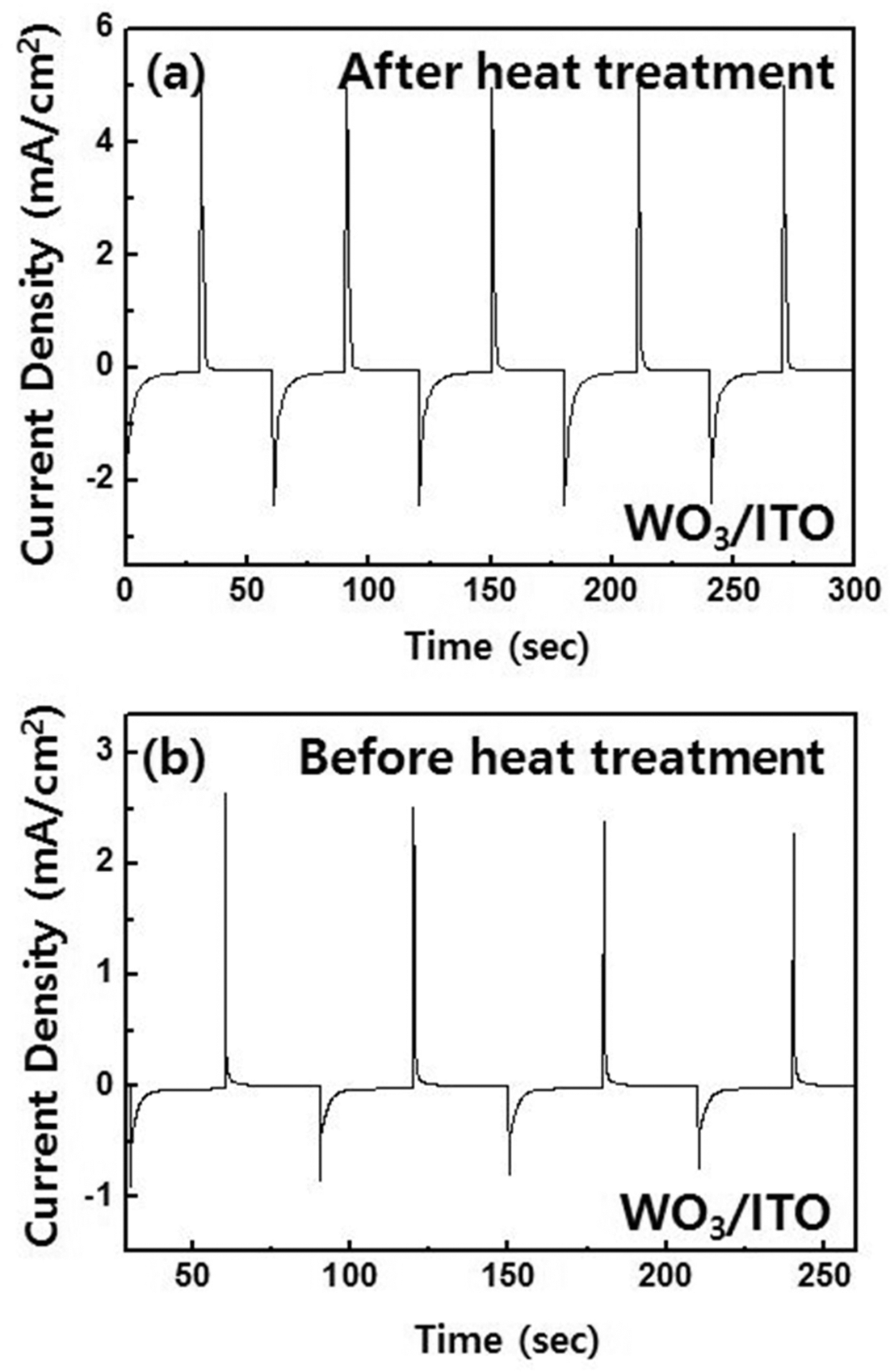
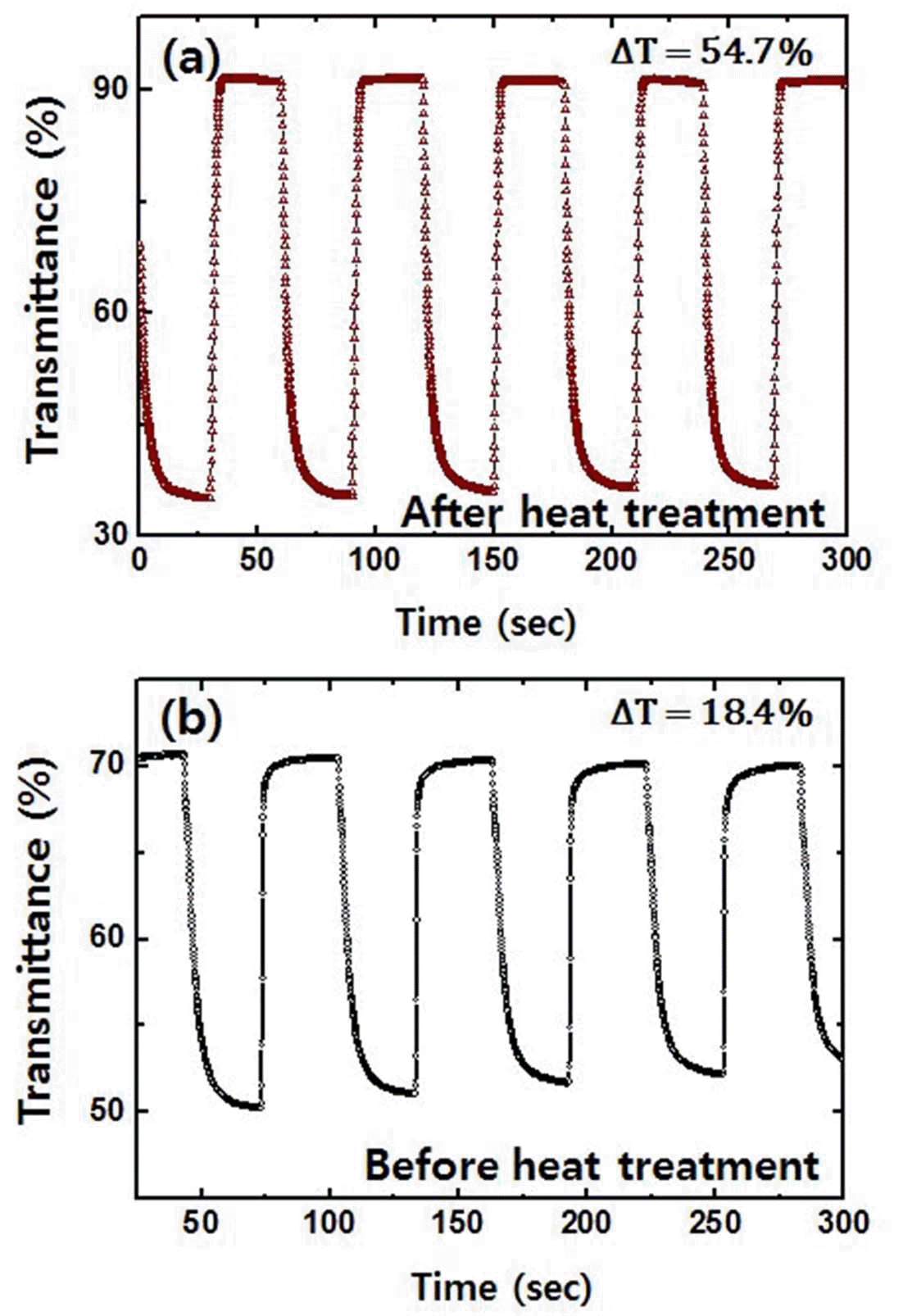
Fig. 1.
SEM images of the surface of the tungsten trioxide films fabricated by the sol-gel method (a) before and (b) after heat treatment. The insets show EDX results.
Fig. 2.
(a) XRD patterns of the silicon substrate and tungsten trioxide/silicon, (b) XPS analysis of tungsten trioxide, and (c) transmittance (absorption in inset) of tungsten trioxide film. The inset is a photograph of a tungsten trioxide film coated on an ITO substrate.
Fig. 3.
The results of cyclic voltammetry, (a) after and (b) before heat treatment of tungsten trioxide film, performed at -0.6 V and 1.0 V with 50 mV/s scan rate. The colored and bleached states of tungsten trioxide are shown in the inset of Fig. 3(a).
Fig. 4.
The results of chronoamperometry performed at -0.6 V and 1.0 V for tungsten trioxide film. (a) after and (b) before heat treatment.
Fig. 5.
Transmittance data collected during chronoamperometry test performed at -0.6 V and 1.0 V for tungsten trioxide film (a) after and (b) before heat treatment.
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Synthesis and Characterization of Tungsten Trioxide Films Prepared by a Sol-Gel Method for Electrochromic Applications
TOP
 KPMI
KPMI

 Cite this Article
Cite this Article





