Articles
- Page Path
- HOME > J Korean Powder Metall Inst > Volume 22(5); 2015 > Article
-
ARTICLE
- Using Carboxylmethylated Cellulose as Water-Borne Binder to Enhance the Electrochemical Properties of Li4Ti5O12-Based Anodes
- Lili Liu, Chongling Chenga, Hongjiang Liu*, Liyi Shi**, Dayang Wanga
-
Journal of Korean Powder Metallurgy Institute 2015;22(5):315-320.
DOI: https://doi.org/10.4150/KPMI.2015.22.5.315
Published online: September 30, 2015
Department of Chemistry, College of Science, Shanghai University, Shanghai 200444, P.R. China
a School of Civil, Environmental, and Chemical Engineering, RMIT University, VIC 3001, Australia
-
*Corresponding author : : Hongjiang Liu, TEL: +61-3-9925-3232,liuhj@shu.edu.cn
**Corresponding author : : Liyi Shi, TEL: +61-3-9925-3232,shiliyi@shu.edu.cn
• Received: October 12, 2015 • Revised: October 20, 2015 • Accepted: October 20, 2015
© The Korean Powder Metallurgy Institute All rights reserved
This is an Open-Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,241 Views
- 8 Download
Abstract
- The present work reports a systematic study of using carboxymethylated cellulose (CMC) as water-borne binder to produce Li4Ti5O12-based anodes for manufacture of high rate performance lithium ion batteries. When the LTO-to-CB-to-CMC mass ratio is carefully optimized to be 8:1:0.57, the special capacity of the resulting electrodes is 144 mAh·g−1 at 10 C and their capacity retention was 97.7% after 1000 cycles at 1 C and 98.5% after 500 cycles at 5 C, respectively. This rate performance is comparable or even better than that of the electrolytes produced using conventional, organic, polyvinylidene fluoride binder.
- Exhaust gas emitted from vehicles is one of the biggest contributions to the increasingly serious environmental pollution nowadays. Over the years, a considerable number of efforts have been put in development of environmental- friendly, green fuels for automobiles instead of fossil fuels [1-3]. In this context, electric vehicles (EVs) and energy storage station (ESS) have been recently developed on the basis of use of high performance lithium ion batteries (LIBs) with long cycle life and high energy density [4-9]. However, the stability and safety of LIBs remain the big technical concerns, which limits the commercialization of LIB [10,11]. To address these issues, novel electrode materials with larger lithium ion storage at high rate have been developed [12].
- Among currently developed anode materials, spinel Li4Ti5O12(LTO) has attracted increasing attention for its outstanding characteristics. Comparing to commercial graphite anodes, spinel LTO exhibits a very flat and relatively higher charge discharge platform at around 1.5 V (versus Li) [13,14]. Thus, it can avoid formation of solid electrolyte interface (SEI) film; otherwise, the latter results in low coulombic efficiency especially in the first formation cycle due to a significant loss of lithium ion [15]. Spinel LTO has negligible volume change during the lithium intercalation/extraction processes(considered as zero-strain material), which results in excellent cycle stability [16,17].
- Typically, LTO electrodes are consisted of active materials (e.g. LTO particles), conductive agents, current collectors and binders. The active materials determine the capacity of the electrode. The conductive agents such as carbon black (CB) are used to improve the rate performance of the electrode owing to the enhanced electric conductivity. The binders are used to glue the active materials and conductive agents together with the current collectors. Thus, the electrons can flow from/to the outside circuit through the active material–conductive agent–current collector chain. Although the mass fraction of the binders are only in the range of 2-10% in a commercial electrode configuration, the binder materials are one of the most crucial electrode components for improvement of the cell performance, especially for cycle life. Without the aid of the binders, the active materials will not properly contact the current collectors, thus resulting in capacity loss [18].
- Nowadays polyvinylidene fluoride (PVDF) is widely used in commercial lithium ion batteries, which is an oily binder and needs to be dissolved in organic solvents such as N-methylpyrrolidone (NMP). Both PVDF and NMP are not environmentally friendly, toxic, and rather expensive. CMC is widely used water-borne binder in many applications [19-22]. Although the use of water for CMC dissolution is environment friendly, obviate strict processing control and allow fast drying. However, a small amount of water left behind in the cell is known to cause a noticeable adverse impact onto the battery performance, especially when LTO is used as anode. New evidences suggests that the use of CMC as binder can manufacture lithium ion batteries such as Si [23-25], Sn [26-28], LiCoO2 [29], and LiFePO4 [30,31], with comparable or better performance than those batteries, in which PVDF is used as binder. In this work, we manufactured LTO anodes, using CMC as binders and carefully studied the impact of the CMC content in the cells on the performance of the resulting LTO anodes. We demonstrate that when the mass ratio of LTO to CMC to CB is 8:1:0.57, the resulting LTO anodes exhibit excellent high rate performance with high capacity. Their specific capacity can be 144 mAh·g–1 at 10 C and the capacity retention is 97.7% after 1000 cycles at 1 C and 98.5% after 500 cycles at 5 C, respectively.
Introduction
- All the reagents were purchased from China National Medicines Corp., Ltd without any purification
- 2.1 Synthesis of LTO particles
- LTO particles were synthesized according to the protocol described in our previous report [32]. Typically, 1.010 g of TiO2 (anatase) and 0.502 g of LiOH·H2O were added into 30 mL of deionized water, followed by magnetic stirring for 30 min. The mixture dispersion was transferred into a Teflon-lined autoclave, followed by incubation at 180°C for 24 h. The precipitates were collected from the bottom of the autoclave by centrifugation. After 3 times repetitions of the centrifugation/water washing cycle and 3 times repetition of the centrifugation/ absolute ethanol washing cycle, the precipitates were dried in 80°C and then underwent calcination at 750°C for 2 h in a nitrogen atmosphere, yielding LTO particles.
- 2.2 Material characterization
- The crystalline structure of the resulting LTO particles was characterized by powder X-ray diffraction (XRD) using D/max 2200 with Cu K (1.54Å) radiation in the 2θ range of 10-70°. The morphology and crystalline structure of the LTO particles were characterized by scanning electron microscopy (SEM) using JSM-6700F (Japan) and transmission electron microscope (TEM) using JEM- 2010F (Japan).
- The electrochemical performance of the resulting LTO anodes was investigated by the assembled CR2016 cointype cells in an argon-filled glove box (MBraun, Germany). The lithium plate was used as the counter electrode. To manufacture the working electrode, CB, and CMC were homogeneously mixed in deionized water with the aid of magnetic stirring for 24 h. Then the resulting slurry was uniformly cast on copper foil and dried at 120°C under vacuum for 12 h. LiPF6 was dissolved in the mixture of ethylene carbonate and dimethyl carbonate (1/1 in volume) at the concentration of 1 M, which was used as the lithium ion battery electrolyte. Following the similar protocol, the PVDF was used as binder to produce LTO anodes, in which the mass ratio of LTO to CB to PVDF was 8:1:1.
- The coin cells were cycled with different current densities in the voltage window from 1.0 to 2.5 V (vs. Li/Li+) on a CT2001A cell test instrument (LAND Electronic Co. Ltd) at room temperature. The specific capacity of LTO electrode was calculated with respects to the mass of the active materials, i.e. LTO particles. Electrochemical impedance spectroscopy (EIS) was carried out in the frequency range from 100 kHz to 0.01 Hz with an electrochemical workstation (CHI660d).
Experimental Section
- Fig. 1(a) shows the XRD pattern of the resulting LTO particles, in which all the diffraction peaks are in good agreement with the spinel LTO phase (JCPDS Card No.49-0207). SEM and TEM imaging indicate that the sizes of the resulting LTO particles are ca. 120 nm (Figs. 1(b) and 1(c). The HRTEM image of the LTO particles also confirms the highly crystalline nature of the resulting LTO particles.
- The rate performance of LTO electrodes obtained by using water-borne CMC binder and organic PVDF binder, respectively, is compared in Fig. 2(a). The specific capacity of the LTO electrodes obtained using the PVDF binder is 171, 168, 162, 160, 157, 152, and 140 mAh·g–1 at 0.1 C, 0.2 C, 0.5 C, 1 C, 2 C, 5 C, and 10 C, respectively. The LTO electrodes obtained using the CMC binder can exhibit specific capacity comparable or slightly larger than those of the LTO electrodes obtained using the PVDF binder, when the LTO-to-CB-to-CMC mass ratio is optimized to be 8:1:0.57. The specific capacity of the resulting electrode is 174, 173, 165, 162, 160, 155, and 144 mAh·g–1 at 0.1 C, 0.2 C, 0.5 C, 1 C, 2 C, 5 C, and 10 C, respectively. As shown in Fig. 2(b), the use of CMC binder results in 10 times increased peel strength of the LTO electrodes.
- Fig. 3 shows the cycling stabilities of the resulting LTO electrodes at 1 C and 5 C. LTO electrodes obtained using the PVDF binder retain 90.3% of the specific capacity after 1000 cycles at 1 C (164.8 mAh·g–1 for the 1st cycle and 148.8 mAh·g–1 for the 1000th cycle) and 95% at 5 C (147.9 mAh·g–1 for the 1st cycle and 140.5 mAh·g–1 for the 500th cycle). In comparison, LTO electrodes obtained using the CMC binder exhibit a slight better cycling stability, which retain >97.7% of their specific capacity retains after 1000 cycles at 1 C (162.4 mAh·g–1 for the 1st cycle and 158.7 mAh·g–1 for the 1000th cycle) and 98.5% after 500 cycles at 5 C(153.3 mAh·g–1 for the 1st cycle and 151 mAh·g–1 for the 500th cycle). This small but non- negligible difference in the cycling stability should reflect the difference in the adhesion between the current collector and activity materials between the LTO electrodes obtained using CMC and PVDF binders (indicated in Fig. 2(b)).
- The rate performances of the LTO electrodes obtained at different LTO-to-CB-to-CMC mass ratios are showed in Figs. 4(a) and 4(b). Note that the LTO-CB mass ratio is set as 8:1 for all the electrodes for easy comparison. The results show that the rate performance is initially enhanced with the CMC content decreasing, while noticeably deteriorates after the CMC content decreases below 0.50. The optimal LTO-to-CB-to-CMC mass ratio is found to be 8:1: 0.57 to achieve the largest specific capacity.
- In order to evaluate the effect of impedance on electrochemical performance, The electrochemical impedance spectra (EIS) enables study of the effect of impedance on the electrochemical performance of LTO electrode, which comprise a semicircle in the high frequency range and a sloping line in the low frequency range, which can be ascribed to the charge transfer coupled with a capacitance at the interface of the LTO particles for the former and the lithium ion diffusion in the bulk of LTO material for the latter. Based on the EIS spectra, one can deduce the equivalent circuits can be deduced, where Rs represents the ohmic resistance including total resistance of the electrolyte, separator and electrical contacts, Rct the charge-transfer resistance, CPE the constant phase-angle element involving double layer capacitance, and Ws (Warburg impedance) the solid-state diffusion of Li+ in the bulk of the active material. Fig. 4(d) shows the electrochemical impedance spectra(EIS) of the LTO electrodes, obtained using CMC and PVDF binders, at a stable voltage of 1.56V. It reveal that Rct of the LTO electrodes obtained using CMC binder is approximately 18Ω, slightly smaller than that of the LTO electrodes obtained using PVDF binder (24Ω) .
Results and Discussion
Fig. 2.
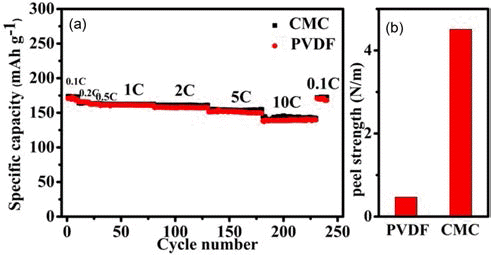
(a) Plots of the rate performance of the LTO electrodes, which are produced by using CMC (red solid dots) and PVDF (black solid squares) binders, respectively, versus the cycle number. The LTO-to-CB-to-CMC mass ratio is optimized to be 8:1:0.57. The LTO-to CB-to-PVDF mass ratio is 8:1:1 and (b) The peel strength of these electrodes.

Fig. 3.
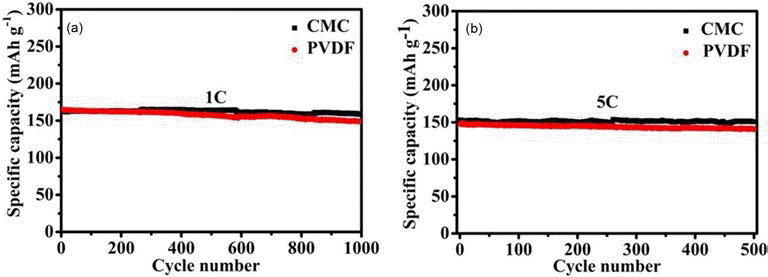
Plots of the cycle performance of the LTO electrodes obtained using CMC (black solid squares) and PVDF (red solid circles) binder versus the cycle number at 1 C, (a) and 5 C and (b). The LTO-to-CB-to-CMC mass ratio is optimized to be 8:1:0.57. The LTO-to-CB-to-PVDF mass ratio is 8:1:1.

Fig. 4.
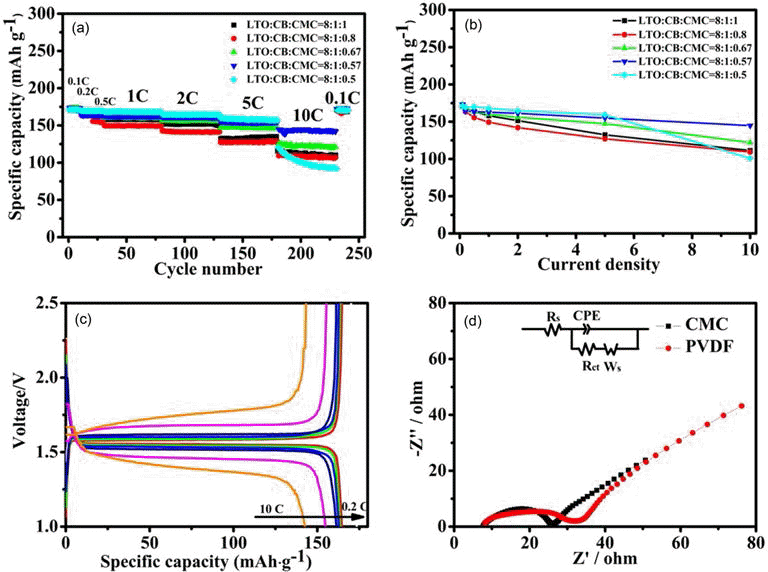
(a) Plots of the rate performance of LTO electrodes with CMC binder versus LTO-to-CB-to-CMC mass ratios: 8:1:1 (black solid squares), 8:1:0.67 (green up triangles), 8:1:0.80 (red solid dots), 8:1:0.57 (blue down triangles), 8:1:0.50 (cyan solid dots), (b) Plot of the specific capacity of the LTO electrodes, obtained at the LTO-to-CB-to-CMC mass ratio of 8:1:1 (black curve), 8:1:0.8(red curve), 8:1:0.67 (green curve), 8:1: 0.57 (blue curve), and 8:1:0.5 (cyan curve), versus current rate, (c) Charging/discharging flat curves of the LTO electrode, obtained at the LTO-to-CB-to-CMC mass ratio of 8:1:0.57, in different current rate and (d) Electrochemical impedance spectra of the LTO electrodes, obtained using CMC(black solid squares) and PVDF binders (red solid dots), at a voltage of 1.56 V. the LTO-to-CB-to-CMC mass ratio is 8:1:0.57, while the LTO-to-CB-to- PVDF mass ratio is 8:1:1. The equivalent circuit is depicted in the inset, in which Rs is the resistance of the electrolyte, Rct the charge transfer resistance, Zw the Warburg impedance and CPE the constant phase element.

- In this study, we demonstrate that the use of waterborne CMC binder can produce LTO electrodes with rate performance comparable to or even better than those produced by using PDVF binders, after careful optimization of the LTO-to-CB-to-CMC mass ratio. In our work, the optimal LTO-to-CB-to-CMC mass ratio is found to be 8:1:0.57. The resulting LTO electrodes show the specific capacity of 174 mAh·g–1 at 0.1 C and 144 mAh·g–1 at 10 C. The electrodes can remain 97.7% of the original specific capacity up to 1000 cycles at 1 C and 98.5% up to 500 cycles at 5C, underlining a good cycling stability. Thus, the present work demonstrates that by proper optimization, environmentally friendly water-borne binders such as CMC can be used as binder for lithium ion batteries instead of organic PVDF binder.
Conclusions
-
Acknowledgements
- Pengfei Hu and Jianchao Peng are acknowledged for assistance with HRTEM characterization and Professional and Technical Service Platform for Designing and Manufacturing of Advanced Composite Materials, Shanghai. This work was financially supported by Shanghai Municipal Science and Technology Commission (13dz2292100) and Baoshan District Science and Technology Commission of Shanghai (bkw2013142).
Acknowledgements
- 1. JM Tarascon and M Armand, Nature. (2001) 414 –359.
- 2. J Cabana, L Monconduit, D Larcher and MR Palacín, Adv Mater. (2010) 22 –E170.
- 3. PG Bruce, B Scrosati and JM Tarascon, Angew Chem. Int. Ed. (2008) 47 –2930.
- 4. V Etacheri, R Marom, R Elazari, G Salitra and D Aurbach, Energy Environ Sci. (2011) 4 –3243.
- 5. JB Goodenough, Energy Environ Sci. (2014) 7 –14.
- 6. A Nugroho, SJ Kim, KY Chung and J Kim, Electrochim Acta. (2012) 78 –623.
- 7. J Huang and Z Jiang, Electrochim Acta. (2008) 53 –7756.
- 8. Y Wang and G Cao, Adv Mater. (2008) 20 –2251.
- 9. J Hassoun, S Panero, P Simon, PL Taberna and B Scrosati, Adv Mater. (2007) 19 –1632.
- 10. Z Cui, F Gao, Z Cui and J Qu, J Power Sources. (2012) 207 –150.
- 11. A Ito, D Li, Y Ohsawa and Y Sato, J Power Sources. (2008) 183 –344.
- 12. BL Ellis, WRM Makahnouk, Y Makimura, K Toghill and LF Nazar, Nat Mater. (2007) 6 –749.
- 13. TF Yi, LJ Jiang, J Shu, CB Yue, RS Zhu and HB Qiao, J Phys. Chem. Solids. (2010) 71 –1236.
- 14. M Broussely and G Archdale, J Power Sources. (2004) 136 –386.
- 15. L Shen, C Yuan, H Luo, X Zhang, K Xu and F Zhang, J Mater. Chem. (2011) 21 –761.
- 16. J Haetge, P Hartmann, K Brezesinski, J Janek and T Brezesinski, Chem Mater. (2011) 23 –4384.
- 17. N Li, G Zhou, F Li, L Wen and HM Cheng, Adv Funct. Mater. (2013) 23 –5429.
- 18. SL Chou, Y Pan, JZ Wang, HK Liu and SX Dou, Phys Chem. Chem. Phys. (2014) 16 –20347.
- 19. C Mangwandi, L JiangTao, AB Albadarin, RM Dhenge and GM Walker, Powder Technol. (2015) 270 –424.
- 20. AA Dangi and PB Zalodiya, Int J. Pharm. Investig. (2012) 2 –183.
- 21. C Hüttl, C Hettrich, R Miller, BR Paulke, P Henklein, H Rawel and FF Bier, BMC Biotechnol. (2013) 13 –1.
- 22. US Vogl, PK Das, AZ Weber, M Winter, R Kostecki and SF Lux, Langmuir. (2014) 30 –10299.
- 23. B Lestriez, S Bahri, I Sandu, L Roué and D Guyomard, Electrochem Commun. (2007) 9 –2801.
- 24. SL Chou, JZ Wang, M Choucair, HK Liu, JA Stride and SX Dou, Electrochem Commun. (2010) 12 –303.
- 25. ZJ Han, N Yabuuchi, K Shimomura, M Murase, H Yui and S Komaba, Energy Environ Sci. (2012) 5 –9014.
- 26. SL Chou, JZ Wang, C Zhong, MM Rahman, HK Liu and SX Dou, Electrochim Acta. (2009) 54 –7519.
- 27. J Li, DB Le, PP Ferguson and JR Dahn, Electrochim Acta. (2010) 55 –2991.
- 28. SL Chou, XW Gao, JZ Wang, D Wexler, ZX Wang, LQ Chen and HK Liu, Dalton trans. (2011) 40 –12801.
- 29. CC Li, JT Lee, YL Tung and CR Yang, J Mater. Sci. (2007) 42 –5773.
- 30. A Guerfi, M Kaneko, M Petitclerc, M Mori and K Zaghib, J Power Sources. (2007) 163 –1047.
- 31. W Porcher, P Moreau, B Lestriez, S Jouanneau, F LeCras and D Guyomard, Ionics. (2008) 14 –583.
- 32. C Cheng, H Liu, J Li, X Xue, H Cao, D Wang and L Shi, J Power Sources. (2015) 283 –237.
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

Using Carboxylmethylated Cellulose as Water-Borne Binder to Enhance the Electrochemical Properties of Li4Ti5O12-Based Anodes
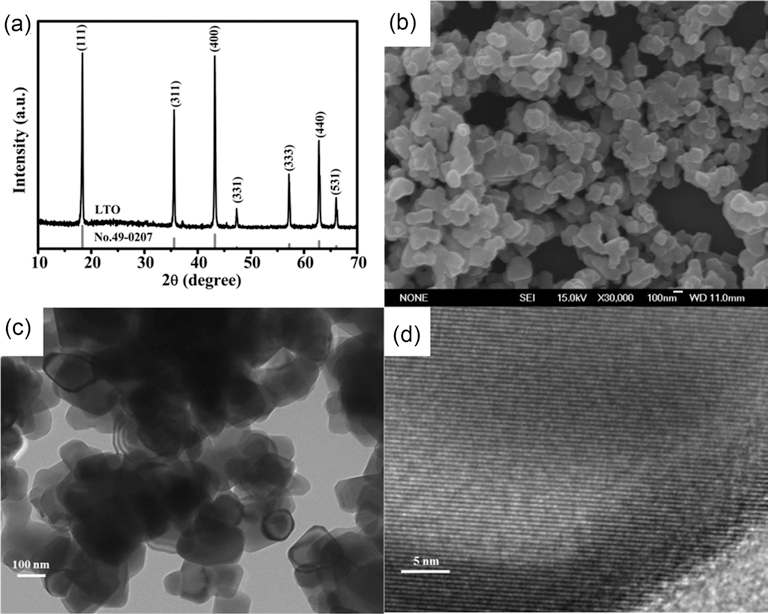
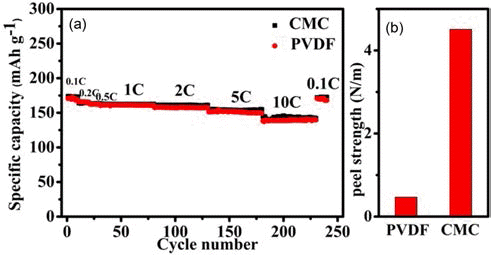

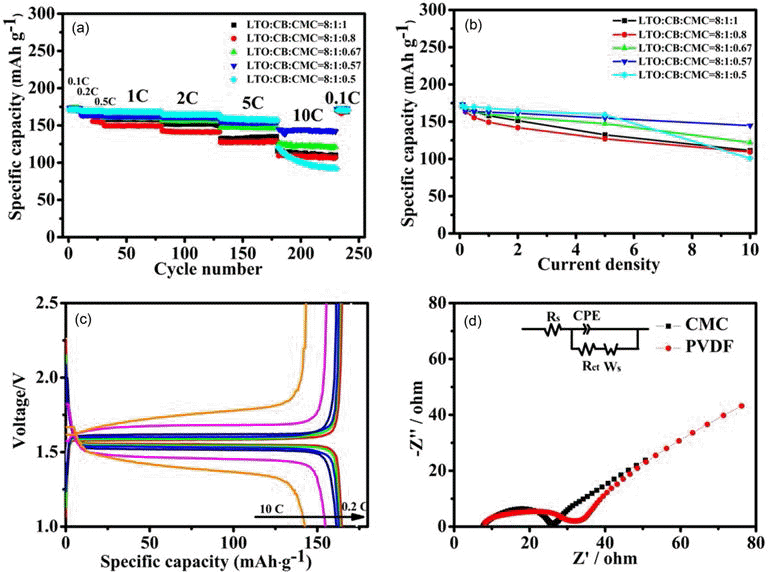
Fig. 1.
(a) XRD pattern and (b) SEM, (c) TEM, and (d) HRTEM images of the LTO particles obtained.
Fig. 2.
(a) Plots of the rate performance of the LTO electrodes, which are produced by using CMC (red solid dots) and PVDF (black solid squares) binders, respectively, versus the cycle number. The LTO-to-CB-to-CMC mass ratio is optimized to be 8:1:0.57. The LTO-to CB-to-PVDF mass ratio is 8:1:1 and (b) The peel strength of these electrodes.
Fig. 3.
Plots of the cycle performance of the LTO electrodes obtained using CMC (black solid squares) and PVDF (red solid circles) binder versus the cycle number at 1 C, (a) and 5 C and (b). The LTO-to-CB-to-CMC mass ratio is optimized to be 8:1:0.57. The LTO-to-CB-to-PVDF mass ratio is 8:1:1.
Fig. 4.
(a) Plots of the rate performance of LTO electrodes with CMC binder versus LTO-to-CB-to-CMC mass ratios: 8:1:1 (black solid squares), 8:1:0.67 (green up triangles), 8:1:0.80 (red solid dots), 8:1:0.57 (blue down triangles), 8:1:0.50 (cyan solid dots), (b) Plot of the specific capacity of the LTO electrodes, obtained at the LTO-to-CB-to-CMC mass ratio of 8:1:1 (black curve), 8:1:0.8(red curve), 8:1:0.67 (green curve), 8:1: 0.57 (blue curve), and 8:1:0.5 (cyan curve), versus current rate, (c) Charging/discharging flat curves of the LTO electrode, obtained at the LTO-to-CB-to-CMC mass ratio of 8:1:0.57, in different current rate and (d) Electrochemical impedance spectra of the LTO electrodes, obtained using CMC(black solid squares) and PVDF binders (red solid dots), at a voltage of 1.56 V. the LTO-to-CB-to-CMC mass ratio is 8:1:0.57, while the LTO-to-CB-to- PVDF mass ratio is 8:1:1. The equivalent circuit is depicted in the inset, in which Rs is the resistance of the electrolyte, Rct the charge transfer resistance, Zw the Warburg impedance and CPE the constant phase element.
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Using Carboxylmethylated Cellulose as Water-Borne Binder to Enhance the Electrochemical Properties of Li4Ti5O12-Based Anodes
TOP
 KPMI
KPMI


 Cite this Article
Cite this Article




