Articles
- Page Path
- HOME > J Korean Powder Metall Inst > Volume 24(3); 2017 > Article
-
ARTICLE
- Dispersion Behavior and Size Analysis of Thermally Purified High Pressure-high Temperature Synthesized Nanodiamond Particles
- Hansang Kwona,b,*, Jehong Parkb, Marc Leparouxc
-
Journal of Korean Powder Metallurgy Institute 2017;24(3):216-222.
DOI: https://doi.org/10.4150/KPMI.2017.24.3.216
Published online: May 31, 2017
b Department of materials system engineering, Pukyoung National University, 365 Sinseon-ro, Nam-gu,Busan 48547, Republic of Korea
b Department of R&D, Next Generation Materials Co., Ltd, 365 Sinseon-ro, Nam-gu, Busan 48547, Republic of Korea
c Laboratory of Advanced Materials Processing, EMPA-Swiss federal laboratories for materials scienceand technology, Feuerwerkerstrasse 39, 3602 Thun, Switzerland
- *Corresponding Author: Hansang Kwon, +82-51-629-6383, +82-51-629-6373, kwon13@pknu.ac.kr
• Received: May 2, 2017 • Revised: May 12, 2017 • Accepted: May 16, 2017
© The Korean Powder Metallurgy Institute. All rights reserved.
- 1,320 Views
- 4 Download
- 1 Crossref
Abstract
- Synthesized monocrystalline nanodiamond (nD) particles are heat-treated at various temperatures to produce highly structured diamond crystals. The heat-treated nDs show different weight loss ratios during thermogravimetric analysis. The crystallinities of the heat-treated nDs are analyzed using Raman spectroscopy. The average particle sizes of the heat-treated nDs are measured by a dynamic light scattering (DLS) system and direct imaging observation methods. Moreover, individual dispersion behaviors of the heat-treated nD particles are investigated based on ultrasonic dispersion methods. The average particle sizes of the dispersed nDs according to the two different measurement methods show very similar size distributions. Thus, it is possible to produce highly crystallized nD powder particles by a heattreatment process, and the nD particles are relatively easy to disperse individually without any dispersant. The heattreated nDs can lead to potential applications such as in nanocomposites, quantum dots, and biomedical materials.
- Nanodiamonds (nDs) have been developed as additives to optimize mechanical and thermal performance in composites, optics, electronics, and biomedical applications due to their superior properties, such as high hardness (103±22 GPa and 83±15 GPa at smaller loads of 30 and 50 mN), excellent thermal conductivity (~2200W·m−1·K−1), lower coefficients of thermal expansion (0.8×10−6 oK−1 at 25°C), large surface area (200~450 m2/g), easy surface functionalization, chemical stability, and non-toxicity [1-5]. nD particles were first produced by a detonation method in the USSR in the 1960s [6, 7].
- In general, they have been synthesized by detonation techniques [7, 8], laser ablation [9], high-pressure high-temperature (HPHT) methods [10], plasma-assisted chemical vapor deposition (CVD) [11], autoclave synthesis from supercritical fluids [12], chlorination of carbides [13], ion irradiation of graphite [14], electron irradiation of carbon “onions” [15], and ultrasound cavitation [16]. Today, the detonation, HPHT, and laser ablation methods are being used commercially.
- Recently, many researchers are investigating the possibilities of advanced atomic-level composites reinforced with nDs [17], drug delivery media [18], and better optical application materials [19]. It is worth noting that the technology leading to the individual dispersion of purified nDs under a controlled environment plays an important role in the realization of nD applications. Moreover, surface chemistry and dispersion of the nD powders are important issues in order to apply for engineering application.
- Air oxidation is simple and inexpensive and does not require the use of any toxic substance or a specific cata- lyst [20]. Several research works was reported which are related size modification [21-24] and purity control [25-27] by air oxidation process. Those valuable results served possibility of highly purified nD powders as industrial applications [28].
- Despite the many previous research works, there is still not a successfully report on the perfect individual dispersion of nDs in a simple method [29]. However, the dispersion of nanoparticles including nD particles is still a major problem in nanotechnology.
- In this study, we have attempted to establish the control of the dispersion quality of thermally purified nD particles by a simple processing method, and the average particle size of the nDs was analyzed by effective measuring methods. First, the HPHT-synthesized monocrystalline nDs were heat-treated in a muffle furnace to produce high quality nD particles. Subsequently, the purified nD particles were dispersed by ultrasonification through pipet and aerosol sampler and onto a Si wafer to examine their dispersities and average particle size by optical image analyzing method. Moreover, the average particle size distribution of the heat-treated nD particles was analyzed by dynamic light scattering (DLS) of calculated the surface area of the individual nD particles. The obtained two average particle size of nD by different analysis method was discussed.
Introduction
- Synthesized nanodiamonds (nDs) by the HPHT method were used as the raw material (Reishauer AG, Switzerland, average particle size of 125 nm) [30]. Thermogravimetric analysis (model: TGA7 Thermogravimetric Analyzer, Perkin Elmer, USA) was carried out to examine the purification of the nD particles at various temperatures (up to ~800°C) and holding times (~300 min.) in an air (78% N2 and 22% O2) atmosphere with a 5°C/min heating rate. Moreover, a relatively high amount of the nDs was heattreated by a muffle furnace (model: L5/12, Nabertherm Gmbh, Germany) at 600°C for 300 min with a 5°C/min heating rate. Raman spectroscopy was performed using a red He-Ne ion laser with a wavelength of 633 nm (Leica, Switzerland) to evaluate the quality of nDs. The microstructure and elements of the nDs was observed by high resolution cold field emission scanning electron microscopy and Energy-dispersive X-ray spectroscopy (Hitachi, HRCFE-SEM-EDS S-4800). The heat-treated nD particles (0.1 g) were dispersed by ultrasonication (model: UP50H, Hielscher Ultrasonic Gmbh, Germany) in 10 ml of water for 5 min under room temperature. Then, the ultrasonically dispersed solution was directly dropped using pipettes onto a polished Si wafer and dried. Furthermore, the ultrasonicated heat-treated nDs were directly dispersed onto the polished Si wafer by using a nanometer aerosol sampler (model: TSI 3089, TSI Incorporated, USA) based on electrostatic method [31]. Both dispersed nDs were observed under SEM to examine the dispersion effectiveness, and then the average particle sizes of the individually dispersed nDs were optically analyzed based on the SEM images by a PowderShape analysis system (model: PowderShape MF, IST-AG-Innovative Sintering Technologies, Switzerland) [32]. The average particle size distribution of the heattreated nDs was also directly analyzed by dynamic light scattering DLS (model: StabiSizer PMX200C, Microtrac Europe Gmbh, Germany) [33].
Experimental
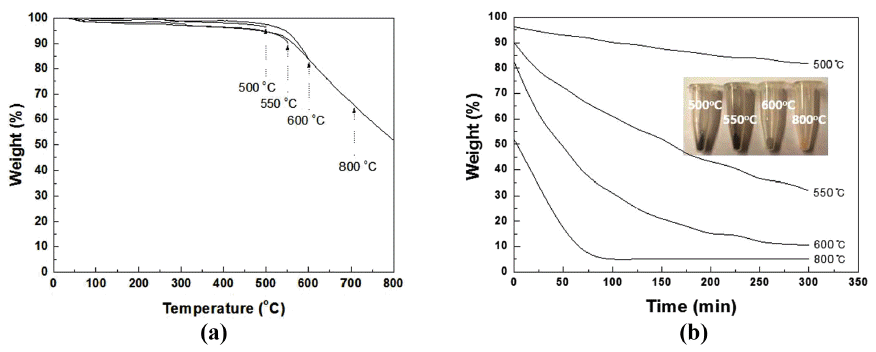
- As shown in Fig. 1 in the SEM-EDS analysis, the assynthesized monocrystalline nDs were highly agglomerated with an irregular morphology and contained other allotropic carbon impurities. The EDS results showed that a relatively high amount of carbon content was contained in the raw nDs; however, the raw nD powder also contained approximately 8.5 wt.% of oxygen. Thermal treatment was used for the purification of nD particles to remove sp2- bonded carbon from the nD particles for further investigation of the dispersion behavior of sp3 nD particles. Thermal gravity analysis (TGA) is widely used for the understanding of thermal behavior of materials. The weight loss of the nD particles showed different values depending on the processing temperatures from the TGA analysis. Approximately 4, 10, 16, and 48% weight losses were found at 500, 550, 600, and 800°C, respectively (Fig. 2a). The weight loss ratios were remarkably changed after 300 min of holding times, except for the temperature of 500°C (Fig. 2b). In particular, the maximal weight loss was found at 800°C at approximately 220 min of holding point, showing 3wt% of remaining nDs, i.e., 97 wt% of nDs was burned out. A temperature of 600°C with a holding time of 300 min seemed to reach near the saturation behavior, and there was still a relatively high amount of nDs (almost 16 wt%) left. This behavior indicated that the processing temperature played an important role in the purification of the nDs, which can be directly related to setting up the heat-treatment temperature of the nDs. Moreover, a brownish white nD powder was obtained at 800°C with a holding time of 300 min, and in the case of 600°C with a holding time of 300 min, a greyish white powder was obtained. In general, the purity of nDs (sp3- bonded carbon) can be simply determined by the color [1, 18, 23, 24]. The brownish white color of the nD powder obtained at 800°C with a holding time of 300 min condition indicated overheating, which produced a high amount of impurities such as carbon onion, fullerenic shell, amorphous carbon, and graphite ribbon [1].
- Raman spectroscopy is very useful method for understanding the quality of nDs. It was observed that the raw synthesized monocrystalline nDs exhibited a relatively high quality because they did not show a defect induced band (D-band) from the Raman spectrum in Fig. 3. For all of the heat-treated samples in this study, as well as the raw nDs, diamond (sp3) peaks were observed. The heattreated sample from 600°C for 300 min did not show a graphitic induced peak (G-band), which is generally observed in sp2-bonded carbon materials (Fig. 3). This means that sp2-bonded carbon in the raw nDs were selectively oxidized to gaseous carbon oxides which were removed from the surface by a simple heat treatment process in an air atmosphere, resulting sp3 peak increased. However, optimized heat-treatment conditions must be applied; otherwise, a significant amount of nD powders will be lost even though high quality sp3-bonded carbon based nD particles can be obtained. Furthermore, the Raman spectrum of the heat-treated sample at 600°C for 300 min showed a rough spectra line and may be due to the fact that this thermal treated sample was still in a transient state even though only the diamond peak was observed. A detailed structural analysis of the purified nDs under optimized conditions is out of the scope of the current study but will be addressed in a future study. According to the experimental results, the Raman spectrum (Fig. 3) of the thermally-purified nDs at 600°C for 300 min showed a high diamond peak intensity without a graphitic peak. Hence, we have selected 600°C with a holding time of 300 min as the optimal conditions for producing high quality and a relatively high amount of purified nD powders in a box furnace.
- The black color of the raw nD powder was changed to a greyish white color, as shown in Figs. 4(a) and (b). The 85% weight loss of the nDs (10 g) after heat treatment in the box furnace was similar to that from the TGA analysis (10 mg), which was shown in Fig. 2. Some fine particles were observed, produced by the thermal decomposition of nDs during the heat treatment (Figs. 4(d) and (e)). However, most of the particle sizes and morphologies of the heat-treated nDs were similar to that of the raw nDs, as shown in Figs. 1 and 4. Hence, heattreatment process did not seriously affect particle growth, which is generally known as a driving force of particle growth. Despite the good quality of the nDs obtained after heat treatment, the yield (collection ratio) was still very low. However, this critical issue can be addressed by a deeper investigation of the effect of temperature for heat-treatment.
- The heat-treated greyish white nD particles after ultrasonication were well dispersed onto the Si wafer by the direct pipette dropping and the electrostatic dispersion through aerosol sampler. Both methods showed similar behaviors, as shown in Fig. 5. In general, the dispersion of nanoparticle materials is the most critical issue due to their large surface area which can lead to strong agglomeration. However, the heat-treated nDs in distilled water without any dispersant can be easily dispersed by ultra- sonication method, which means that distilled water can be used as a good dispersant of the heat-treated nD particles.
- The average particles size and distributions of the purified nD particles prepared by direct pipette and aerosol sampler were measured through particle size analysis instruments of PowderShape (SEM image) and Stabisizer (DLS). Powdershape is a characterization system for powders and all kinds of particles and has been specially developed for quality inspection [32]. Stabisizer is a characterization system for colloids via dynamic light scattering (DLS) [33]. Brief information of these methods was given in the experimental procedure section. The two differently prepared particles exhibited similar D50 values of 80~90 nm from the PowderShape results (Figs. 6(a) and (b)). The range of the distribution of size was from 20~180 nm, and the smallest size of the measured nD particles was found in the aerosol sampler (Fig. 6(b)). Moreover, the aerosol sampler particles seemed to have a better dispersity than the direct pipette dropped one due to a narrower and more continuously distributed histogram. However, within a reasonable error range of both equipment (±5%), it is estimated that both particles exhibited similar dispersities.
- That the D50 value (88 nm) of heat-treated nDs obtained from DLS was very similar to the PowderShape results as shown in Fig. 7. It is worth noting that the size distribution of 100 nm sized nDs can be measured by SEM image analysis equipment (PowderShape) and DLS methods regardless of the heat treatment applied, as indicated in Table 1. In other words, heat-treated nDs were well dispersed in the distilled water without any dispersant during sampling onto the Si wafer indicate individual dispersion of the nDs possible through by ultrasonication process. This possibility offer the further processing of nDs for potential applications in nanocomposites, quantum dots, and biomedical materials, etc.
Results and Discussions
Fig. 1
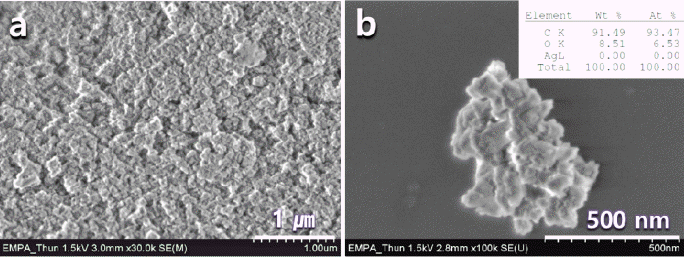
(a) Synthesized Monocrystalline nanodiamond (D90=125 nm) and (b) high resolution of (a). The right upper-inset at (b) shows the composition of nanodiamond.

Fig. 3
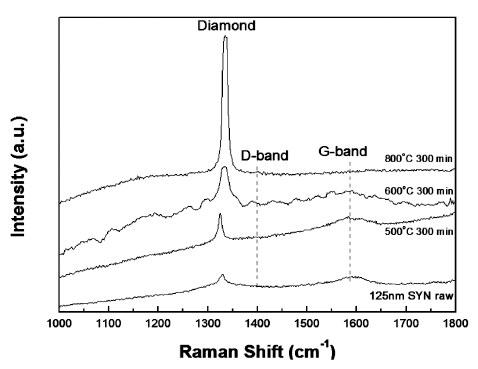
Raman spectrums of the raw nD, and purified nD at 500, 600, and 800°C with 300 min holding time.

Fig. 4

(a) Digital pictures of the before and after heat-treated (600°C 420 min in air) nD powders. (c-f) morphology of the heat-treated nD particles.

Fig. 5
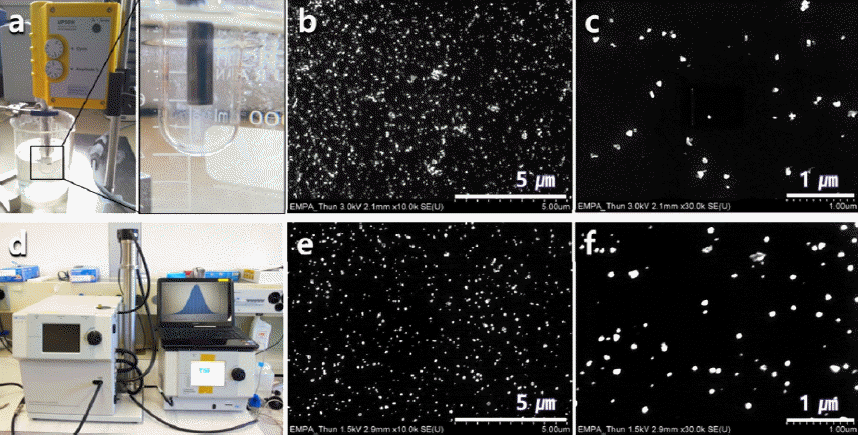
Digital picture of (a) ultrasonic dispersion equipment. (b and c) SEM micrographs of the directly dropped nD particles using pipettes onto a polished Si wafer. Digital picture of (d) electrostatic dispersion equipment and (e and f) SEM micrographs of the electrostatic dispersed -nD particles.

Fig. 6
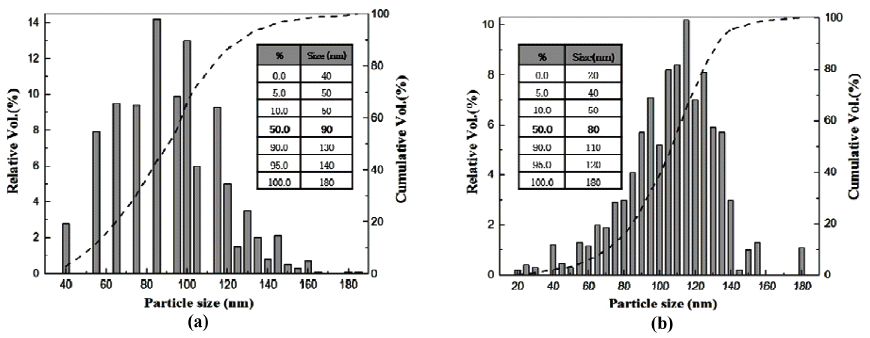
Particle size distribution analysis of purified-nD diapersded by (a) direct pipette and (b) aerosol sampler using a PowderShape equipment.

Fig. 7
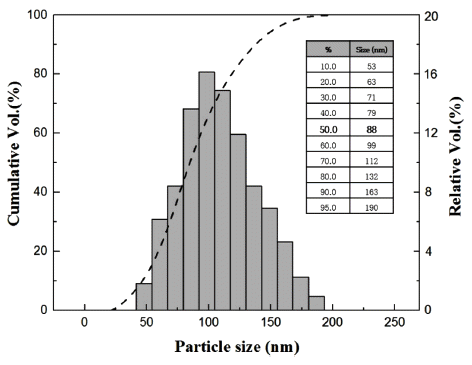
Particle size distribution analysis of purified-nD by a dynamic light scattering (DLS) method.

Table 1
Particle size analysis results of raw nD and purified-nD (600oC, 300 min in air) depending on analysis methods.
- Highly purified nD powder particles were successfully achieved by heat treatment in air atmosphere. The heat treated nD particles were easily dispersed in distilled water without any dispersant by ultrasonication dispersion methods. Average particle sizes of the purified nD particles were shown to be approximately 90 nm regardless different characterized methods by image analysis (PowderShape and Aerosol sampling) and dynamic light scattering (DLS) methods. Moreover, the average particle size of the heat-treated nDs obtained was slightly smaller than that of the raw nD particles (around 120 nm) regardless of the particle size analysis methods. According to the achieved results, the heat-treatment process is an efficient way to achieve nD particles, but the yield is still very low. However, we believe that the low yield problems could be overcome through the optimization of heat-treatment parameters such as precisely controlled temperature, holding time, and atmosphere. We believe that these basic experimental results will facilitate the real application of nD particles in industry.
Conclusions
-
Acknowledgements
- This work was supported by a Research Grant of Pukyong National University (2016 year)
Acknowledgements
- 1. S. Osswald, G. Yushin, V. Mochalin, S.O. Kucheyev and Y. Gogotsi: J. Am. Chem. Soc., (2006) 128 11635.Article
- 2. A. Niederhofer, P. Nesladek, H.D. Mannling, K. Moto and et al: Surf. Coat. Technol., (1999) 120-121 173.Article
- 3. H. Kwon, S. Kim, B. Lee, W. Seo and M. Laparoux: Mater. Sci. Eng. A., (2015) 632 72.Article
- 4. E. Duffy, D.P. Mitev, S.C. Thickett, A.T. Townsend, B. Paul and P.N. Nesterenkon: Appl. Surf. Sci., (2015) 357 397.Article
- 5. X. Zhang, J. Yin, C. Kang, J. Li, Y. Zhu, W. Li, Q. Huang and Z. Zhu: Toxicol. Lett., (2010) 198 237.Article
- 6. V.V. Danilenko: Phys. Solid State., (2004) 46 595.ArticlePDF
- 7. V. Pichot, B. Risse, F. Schnell, J. Mory and D. Spitzer: Sci. Rep., (2013) 3 02159.
- 8. A.R. Kirmani, W. Peng, R. Mahfouz, A. Amassian, Y. Losovyj, H. Idriss and K. Katsiev: Carbon., (2015) 94 79.Article
- 9. G.W. Yang, J.B. Wang and Q.X. Liu: J. Phys. Condens. Matter., (1998) 10 7926.
- 10. J.P. Boudou, P.A. Curmi, F. Jelezko, J. Wrachtrup, P. Aubert, M. Sennour, G. Balasubramanian, R. Reuter, A. Thorel and E. Gaffet: Nanotechnology., (2009) 20 235602.Article
- 11. M. Frenklach, W. Howard, D. Huang, J. Yuan, K.E. Spear and R. Koba: Appl. Phys. Lett., (1991) 59 546.ArticlePDF
- 12. Y. Gogotsi and G. Klaus and et al: J. Mater. Chem., (1996) 6 595.Article
- 13. S. Welz, Y. Gogotsi and M.J. McNallan: J. Appl. Phys., (2003) 93 4207.ArticlePDF
- 14. T.L. Daulton, M.A. Kirk, R.S. Lewis and L.E. Rehn: Nucl. Instrum. Meth. B., (2001) 175 12.
- 15. F. Banhart and P.M. Ajayan: Nature., (1996) 382 433.ArticlePDF
- 16. É.M. Galimov, A.M. Kudin, V.N. Skorobogatskii, V.G. Plotnichenko, O.L. Bondarev, B.G. Zarubin, V.V. Strazdovskii, A.S. Aronin, A.V. Fisenko, I.V. Bykov and A.Yu. Barinov: Dokl. Phys., (2004) 49 150.ArticlePDF
- 17. V.N. Mochalin, I. Neitzel, B.J. Etzold, A. Peterson, G. Palmese and Y. Gogotsi: ACS Nano., (2011) 5 7494.Article
- 18. H. Huang, E. Pierstorff, E. Osawa and D. Ho: Nano Lett., (2007) 11 3305.
- 19. V. Grichko, T. Tyler, V.I. Grishko and O. Shenderova: Nanotechnology., (2008) 19 225201.Article
- 20. T. Gaebel, C. Bradac, J. Chen, J.M. Say, L. Brown, P. Hemmer and J.R. Rabeau: Diamond Related Materials., (2012) 21 28.Article
- 21. S. Osswald, M. Havel, V. Mochalin, G. Yushin and Y. Gogotsi: Diamond Related Materials., (2008) 17 1122.Article
- 22. O.A. Williams, J. Hees, C. Dieker, W. Jager, L. Kirste and C.E. Nebel: ACS Nano., (2010) 8 4824.
- 23. S. Stehlik, M. Varga, M. Ledinsky, V. Jirasek, A. Artemenko, H. Kozak, L. Ondic, V. Skakalova, G. Argentero, T. Pennycook, J.C. Meyer, A. Feifar, A. Kromka and B. Rezek: J. Phys. Chem. C., (2015) 119 27708.Article
- 24. S. Stehlik, M. Varga, M. Ledinsky, D. Miliaieva, H. Kozak, V. Skakalova, C. Mangler, T.J. Pennycook, J.C. Meyer, A. Kromka and B. Rezek: Sci. Rep., (2016) 6 38419.
- 25. O. Shenderova, I. Petrov, J. Walsh, V. Grichko, V. Grishko, T. Tyler and G. Cunningham: Diamond Related Materials., (2006) 15 1799.Article
- 26. V. Pichot, M. Comet, E. Fousson, C. Baras, A. Senger, F. Le Normand and D. Spitzer: Diamond Related Materials., (2008) 17 13.Article
- 27. A. Wolcott, T. Schiros, M.E. Trusheim, E.H. Chen, D. Nordlund, R.E. Diaz, O. Gaathon, D. Englund and J.S. Owen: J. Phys. Chem. C., (2014) 118 26695.Article
- 28. J. Havlik, V. Petrakova, I. Rehor, V. Petrak, M. Gulka, J. Stursa, J. Kucka, J. Ralis, T. Rendlerf, S.Y. Lee, R. Reuter, J. Wrachtrup, M. Ledvina, M. Nesladek and P. Cigler: Nanoscale., (2013) 5 3208.Article
- 29. V.N. Mochalin and Y. Gogotsi: Diamond Related Materials., (2015) 58 161.Article
- 30. Reishauer, Downloadcenter http://rmnt.reishauer.com/en/welcome/downloadcenter
- 31. Y.K. Bahk, J. Buha and J. Wang: Aerosol Sci. Technol., (2013) 47 776.Article
- 32. H.G. Schmid, M. Dvorak, G. Buerki, M. Leparoux, S. Siegmann and C. Schreuders, PARTEC Int. Congress for Particle Technology. (2004) 26 p. 16.
- 33. C. Xu, X. Cai, J. Zhang and L. Liu: Particuology., (2015) 19 82.Article
Figure & Data
References
Citations
Citations to this article as recorded by 

- Two extreme crystal size scales of diamonds, large single crystal and nanocrystal diamonds: Synthesis, properties and their mutual transformation
Yang Wang, Wei-hua Wang, Shi-lin Yang, Guo-yang Shu, Bing Dai, Jia-qi Zhu
New Carbon Materials.2021; 36(3): 512. CrossRef
Dispersion Behavior and Size Analysis of Thermally Purified High Pressure-high Temperature Synthesized Nanodiamond Particles


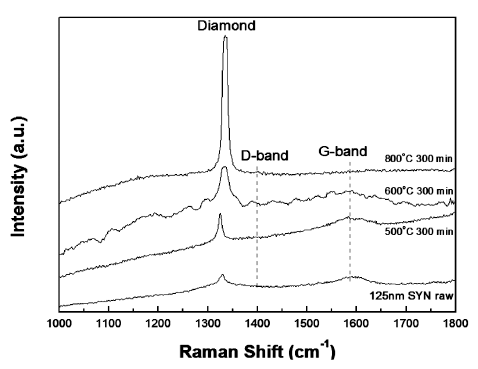




Fig. 1
(a) Synthesized Monocrystalline nanodiamond (D90=125 nm) and (b) high resolution of (a). The right upper-inset at (b) shows the composition of nanodiamond.
Fig. 2
TGA curves of the nanodiamond (a) depending on temperatures with (b) 300 min. holding.
Fig. 3
Raman spectrums of the raw nD, and purified nD at 500, 600, and 800°C with 300 min holding time.
Fig. 4
(a) Digital pictures of the before and after heat-treated (600°C 420 min in air) nD powders. (c-f) morphology of the heat-treated nD particles.
Fig. 5
Digital picture of (a) ultrasonic dispersion equipment. (b and c) SEM micrographs of the directly dropped nD particles using pipettes onto a polished Si wafer. Digital picture of (d) electrostatic dispersion equipment and (e and f) SEM micrographs of the electrostatic dispersed -nD particles.
Fig. 6
Particle size distribution analysis of purified-nD diapersded by (a) direct pipette and (b) aerosol sampler using a PowderShape equipment.
Fig. 7
Particle size distribution analysis of purified-nD by a dynamic light scattering (DLS) method.
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
Fig. 6
Fig. 7
Dispersion Behavior and Size Analysis of Thermally Purified High Pressure-high Temperature Synthesized Nanodiamond Particles
| Sample | Analysis method | Before Heat-treatment (D50%, nm)±5% | After Heat-treatment (D50%, nm)±5% | |
|---|---|---|---|---|
|
|
||||
| Synthesized monocrystalline nanodiamond | SEM Image | (Pipet) | 92 | 90 |
| (Aerosol ampler) | 100 | 80 | ||
| Dynamic light scattering (DLS) | 95 | 88 | ||
Table 1
Particle size analysis results of raw nD and purified-nD (600oC, 300 min in air) depending on analysis methods.
Table 1
TOP
 KPMI
KPMI

 Cite this Article
Cite this Article







