Articles
- Page Path
- HOME > J Korean Powder Metall Inst > Volume 24(3); 2017 > Article
-
ARTICLE
- Magnetically Driven Assemblies of γ-Fe3O4 Nanoparticles into Well-Ordered Permanent Structures
- Myunghwan Byun*
-
Journal of Korean Powder Metallurgy Institute 2017;24(3):229-234.
DOI: https://doi.org/10.4150/KPMI.2017.24.3.229
Published online: May 31, 2017
Department of Advanced Materials Engineering, Keimyung University, Daegu 42601, Republic of Korea
- *Corresponding Author: Myunghwan Byun, +82-53-580-5228, +82-53-580-5165, myunghbyun@kmu.ac.kr
• Received: June 7, 2017 • Revised: June 19, 2017 • Accepted: June 20, 2017
© The Korean Powder Metallurgy Institute. All rights reserved.
- 779 Views
- 1 Download
Abstract
- We report on a simple and robust route to the spontaneous assembly of well-ordered magnetic nanoparticle superstructures by irreversible evaporation of a sessile single droplet of a mixture of a ferrofluid (FF) and a nonmagnetic fluid (NF). The resulting assembled superstructures are seen to form well-packed, vertically arranged columns with diameters of 5~0.7 μm, interparticle spacings of 9~2 μm, and heights of 1.3~3 μm. The assembled superstructures are strongly dependent on both the magnitude of magnetic field and the mixing ratio of the mixture. As the magnitude of the externally applied magnetic field and the mixing ratio of the mixture increase gradually, the size and interspacing of the magnetic nanoparticle aggregations decrease. Without an externally applied magnetic field, featureless patterns are observed for the γ-Fe3O4 nanoparticle aggregations. The proposed approach may lead to a versatile, cost-effective, fast, and scalable fabrication process based on the field-induced self-assembly of magnetic nanoparticles.
- Of the various colloidal suspensions, ferrofluids composed of nanoscale ferromagnetic colloidal particles dispersed in a non-ferromagnetic carrier liquid such as an organic solvent or water have been intensively investigated [1-5]. Evaporative dispersions of ferromagnetic nanoparticles under magnetic field are self-assembled via the combined process of inter-particle interactions such as Van der Waals attractions, dipolar magnetic interactions, and electrostatic or steric repulsions. In the case of ferric oxide nanoparticles, Van der Waals forces lead to an isotropic attractive potential, showing the Hamaker constant with the order of A = 10-19 [7-9]. Anisotropic magnetic dipolar interactions can be strongly dependent upon the angle between the dipolar moment of a particle and the vector joining the centers of two particles, and the azimuthal angle between neighboring dipoles [8-9]. The strength of the magnetic coupling between adjacent particles can be strongly affected by the volume of a particle, the specific magnetization of the element, and the interspacing of particles. Steric repulsive forces originated from each atom within surfactant molecules capped with the nanoparticles lead to energy loss due to overlapping electron clouds when atoms are closely positioned [10-12]. Ferrofluids can be categorized by two distinctive systems; pure ferrofluids and mixtures of a ferrofluid/ non-ferrofluid. Especially, when the latter ferrofluid restricted in a quasi-bidimensional geometry is subjected to an externally applied magnetic field, complex yet intriguing surface instabilities such as hexagonally packed vertical columns or labyrinthine-like parallel stripes prototypically appears [7-13]. Such this morphological evolution is mainly attributed to antagonistic competition between the attractive short range surface tension for minimization of the surface free energy at the domain interface and the repulsive long range dipolar-dipolar interaction at the extended interface.
- Ordered assemblies of ferrofluids composed of magnetic nanoparticles have gained increasing interest due to the rich assembling behavior caused by their reduced shape symmetry and the unique collective properties that can be engineered by controlling dimensional features of nanoparticles including shapes, interparticle spacing, material processing, etc. To date, most studies have been based on assembling behaviors or phase-transitions of the pure ferrofluids or mixtures of ferro/non-ferrofluids confined between two closely parallel plates [7-9] except for a few previous reports related to evaporation of an unbound ferrofluid droplet on a solid flat substrate [14-15]. In the case of the aforementioned approaches, i.e., the pure ferrofluids or an immiscible mixture of a ferrofluid and nonmagnetic fluid, if the externally applied magnetic field is switched off, the characteristic equilibrium structure assembled within a quasi-bidimensional space can be no longer sustained owing to diffusive coarsening process of each phase. Furthermore, visualization of the resulting patterns can be made only by optical microscopy. Once the ferrofluidic domains are assembled at the nanometer scale, they cannot be fully investigated. Although a few studies based on evaporation of a single ferrofluid droplet have been reported to overcome the aforementioned limitations [14-15], there are still challenges to improve the packing order of periodic nanoparticle aggregations and to reduce their agglomeration size by precisely controlling the experimental parameters.
- Herein, we report on a simple and robust route to the spontaneous assemblies of well-ordered magnetic nanoparticle superstructures by irreversible evaporation of a sessile single droplet of a mixture of a ferrofluid (FF) and nonmagnetic fluid (NF). The resulting assembled superstructures formed a well-packed vertical columns with diameter ranging from 5 to 0.7 μm, interparticle spacing ranging from 9 to 2 μm, and height ranging from 1.3 to 3 μm. The assembled superstructures were observed to be strongly dependent upon both the magnitude of magnetic field and mixing ratio of the mixture. As an externally applied magnetic field and the mixing ratio of the mixture increased gradually, the size and interspacing of magnetic nanoparticles aggregations decreased. Without an externally applied magnetic field, featureless patterns of the γ-Fe3O4 nanoparticles aggregations were observed. The proposed approach may open a versatile, cost-effective, fast, and scalable route to the fabrication process based on the field-induced self-assembly of magnetic nanoparticles.
Introduction
- 2.1. Synthesis and Dispersion of γ-Fe3O4 Nanoparticles
- Monodispersed ferromagnetic γ-Fe3O4 nanoparticles were successfully prepared according to well-established procedures [13-15]. The chemical synthesis of ferromagnetic nanoparticles was composed of two steps; synthesis of iron oleate complex and subsequent thermal decomposition of iron oleate. Briefly, a certain amount of iron chloride and sodium oleate (TCI chemicals) were dissolved in the mixture deionized (DI) water, ethanol, and hexane at the volume ratio of 4:3:7 and heated to 70oC for 4hrs. The upper organic phase was extracted using a separation funnel and washed with DI water three times. The iron oleate obtained was sealed and stored in a desiccator. γ-Fe3O4 nanoparticles were obtained by subsequent thermal decomposition of iron oleate in 1-octadecene at 320oC for 30 min, using oleic acid as the surface capping ligand, for improving their solubility in organic solvents. After cooling down to room temperature, the γ-Fe3O4 nanoparticles were purified by adding a large amount of ethanol and re-dissolving in toluene. The resulting magnetic nanoparticles were closely investigated by transmission electron microscopy (TEM) (Fig. 1A). The size of the γ-Fe3O4 nanoparticles stabilized against aggregation with a mixed layer of oleic acid and oleyamine was approximately 8~9 nm as shown in Fig. 1B. A low molecular weight polystyrene was used as a material for capping the nanoparticle, thus avoiding undesired agglomeration in solvent (Inset of Fig. 1A).
- 2.2. Preparation of a Ferro/Non-Ferrofluid Mixture Suspension
- In order to prepare the magnetite ferrofluids, the γ- Fe3O4 nanoparticles were suspended in dodecane as an oily organic solvent. The saturation magnetization value of the particles was found to be 58-59 A2m kg-1, by superconducting quantum interference device (SQUID) magnetometry. The volume fraction of the nanoparticles in dodecane was 15 wt%, which was characterized by the ratio of the volume of dry magnetic power to that of dodecane. This dodecane solution of the particles was referred as the ferrofluid in our experiments. A gel-type triblock copolymer, polystyrene-b-polybutadiene-b-poly styrene (SBS) was selected as the non-ferromagnetic system and was dissolved in toluene. Material choice was motivated by effective entrapment of magnetic nanoparticles aggregations within viscous polymer matrix and minimization of dewetting of polymer matrix during the evaporation process. Subsequently, this SBS toluene solution was mixed with the ferrofluidic suspension of γ- Fe3O4 nanoparticles. The volume ratio of a ferrofluid and non-ferrofluid was 1:3, i.e., forming an asymmetric binary mixture. This suspension was evaporated under externally applied magnetic field as schematically shown in Fig. 1C-D.
- 2.3. Characterization
- The evaporation typically took 5 min to complete, and then the magnetic field was removed. Subsequently, the SBS triblock copolymer matrix was degraded by oxygen plasma treatment for 60 min. Before this removal step, all samples were investigated by optical microscopy (OM) and scanning electron microscopy (SEM) for clear comparison with the surface morphologies without SBS triblock copolymer. Thickness of the samples was measured by Dektak profiler after partial cutting.
Experimental
Fig. 1
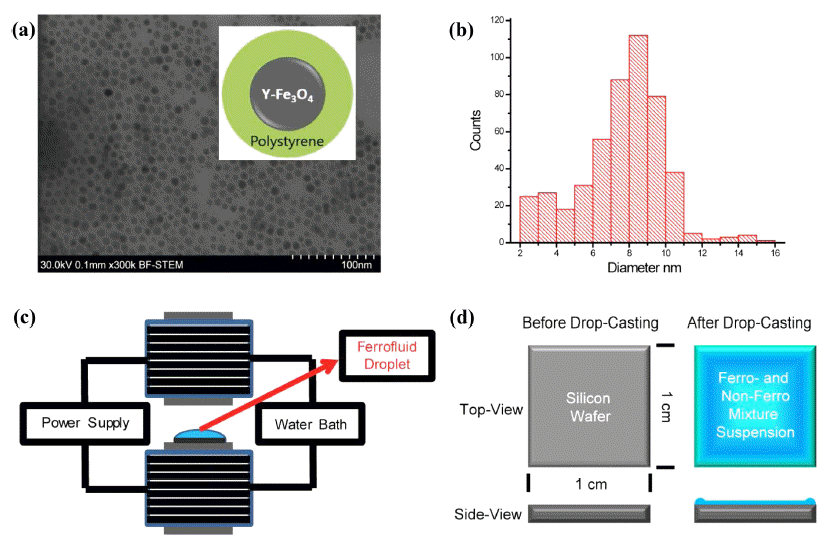
(A) TEM image of the chemically synthesized γ-Fe3O4 nanoparticles. Inset is a schematic cartoon for the γ-Fe3O4 nanoparticle capped with low molecular weight polystyrene. (B) Size distribution of the nanoparticles. (C) A schematic illustration of the externally applied magnetic field during drying process of the unbound sessile single droplet of a mixture of a ferrofluid (FF) and nonmagnetic fluid (NF). (D) A schematic illustration of the unbound sessile single droplet of a mixture of a ferrofluid (FF) and nonmagnetic fluid (NF) before and after drop-casting.

- OM images show that the size and interparticle spacing of the γ-Fe3O4 nanoparticles columns obtained are strongly dependent upon the varying magnitude of a magnetic field (Fig. 2). Every image of the γ-Fe3O4 nanoparticles aggregations under various ranges of magnetic field has been captured from the central regions of the droplet after completed evaporation of toluene and dodecane. At the borders of the silicon wafer, a less uniform film with surface undulations has been instead observed. A set of images in Figure 2 are shown as a function of an externally applied magnetic field. At 25 mT, agglomerations of the γ-Fe3O4 nanoparticles became appeared. As the magnetic field intensity increased from 0 mT to 200 mT, the mean size of the γ-Fe3O4 nanoparticles columns gradually decreased.
- The assembled superstructures were observed to be strongly dependent upon both the magnitude of magnetic field and mixing ratio of the mixture. As an externally applied magnetic field and the mixing ratio of the mixture increased gradually, the size and interspacing of magnetic nanoparticles aggregations decreased. Without an externally applied magnetic field, featureless patterns of the γ-Fe3O4 nanoparticles aggregations were observed (Upper left image of Fig. 2). High resolution FESEM images show that the assembled columns are composed of close-packed the γ-Fe3O4 nanoparticles (Fig. 3A-B). Furthermore, aggregates of capping ligands and disordered clusters of the γ-Fe3O4 nanoparticles have been also observed in the region between the columns. The formation mechanism is deeply based on the competition between evaporation of unbound ferrofluid droplet and externally applied magnetic field. The evaporation speed of the toluene of non-ferrofluid is highly expected to be accelerated compared to dodecane of the carrier of ferrofluid. With increased magnetic field intensity, the column diameter decreased dramatically, whereas the interspacing between adjacent columns decreased and then above 112.5 mT increased. In the presence of the vertically applied magnetostatic field, the dipolar interaction causes a repulsive force among the particles because the dipolar moments are parallel. The resulting assembled superstructures formed a vertically packed columns with diameter of 5~0.7 μm (Fig. 4A), interparticle spacing of 9~2 μm (Fig. 4B), and height of 1.3~3 μm (Fig. 4C). More interestingly, the interspacing between neighboring γ-Fe3O4 nanoparticles columns was observed to be decreased by the range of magnetic field of 112.5 mT, and then showed an increasing tendency from the range of magnetic field of 125 mT to 200 mT. This tendency is probably due to an expected increase in the column repulsion with increasing field strength over the critical limit.
- The possible assembly mechanism can be strengthened by the relaxation processes of a ferrofluid unbound droplet evaporating under magnetic field. The relaxation consists of two processes; 1. The rotation of the single domain particle, which is deeply related to the Brownian motion (i.e., random movements of particles suspended in an evaporative fluid) of the magnetic nanoparticles, 2. Magnetization vector rotation under minimized Brownian motion and immobilized particles. With the evaporating of solvent and shrinkage of the liquid surface, the suspension viscosity becomes greater and the clusters also became larger as the relaxation time increased. However, as the vertical magnetic field increased, the size of columns decreased due to increased repulsive force between adjacent columns. The magnetic force of a magnetic nanoparticle under a static magnetic field can be approximately defined as follows.
- V is the volume of the particle (~μm; equivalently corresponding to the diameter of the spherical particle), Δγ is the difference in the magnetic susceptibilities of the particle, μ0 is the vacuum permeability (~4π × 10-7 J/mA-2) is the magnetic field strength (~T; Tesla or V·s/m2) and BΔ is the field gradient. The evaporation typically took 5 min to complete, after which the magnetic field was removed.
- For comparison of the experimental observation of the equilibrium aggregation diameter with its theoretical prediction, we considered the total energy of a single aggregation estimated as a cylindrical column of diameter (~μm) and height (~μm), interacting with similar aggregations in a closely packed hexagonal array [1, 7]. This consideration can be expressed as
- Here, NB is the dimensionless magnetic Bond number, χ is the magnetic susceptibility (~m3·mol-1), d(d = d0+d1) is the demagnetizing field and σ is the interfacial tension (~J·m2). This equation can fruitful information about morphological evolution of the nanoparticles aggregations under magnetic field. The term of d0 defines the attenuation of the externally applied field arising from dipolardipolar interactions within a single aggregation and the term of d1 indicates the interactions between adjacent aggregations. Hence, the former is a function of the aggregation aspect ratio (D/H), finally showing the maximized status for extended sheet, whereas the latter provides the information on the packing density of the aggregations (i.e., interspacing between neighboring aggregations). Further numerical analysis on the resulting assembled superstructures is now under close investigation.
Results and Discussion
Fig. 2
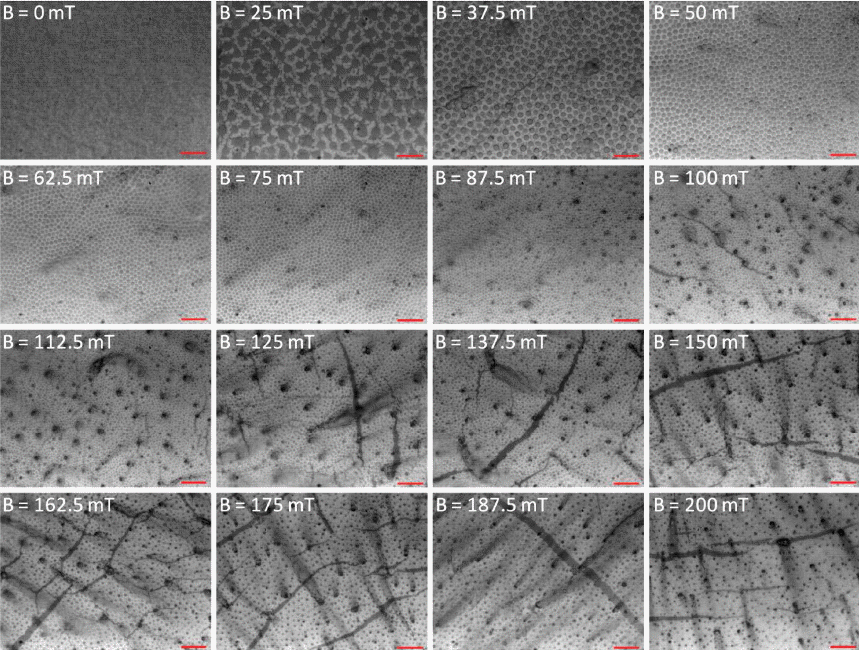
Morphological evolution of the magnetically driven γ-Fe3O4 nanoparticles aggregations with varying magnetic field intensity. Changes of the lateral width and interspacing of the nanoparticle aggregations with varying magnetic field intensity. All scale bars are 10 μm.

Fig. 3
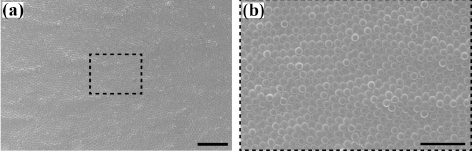
High resolution FESEM images showing the assembled columns composed of close-packed the γ-Fe3O4 nanoparticles aggregations. The scale bars are 20 μm for A and 10 μm for B, respectively.

Fig. 4
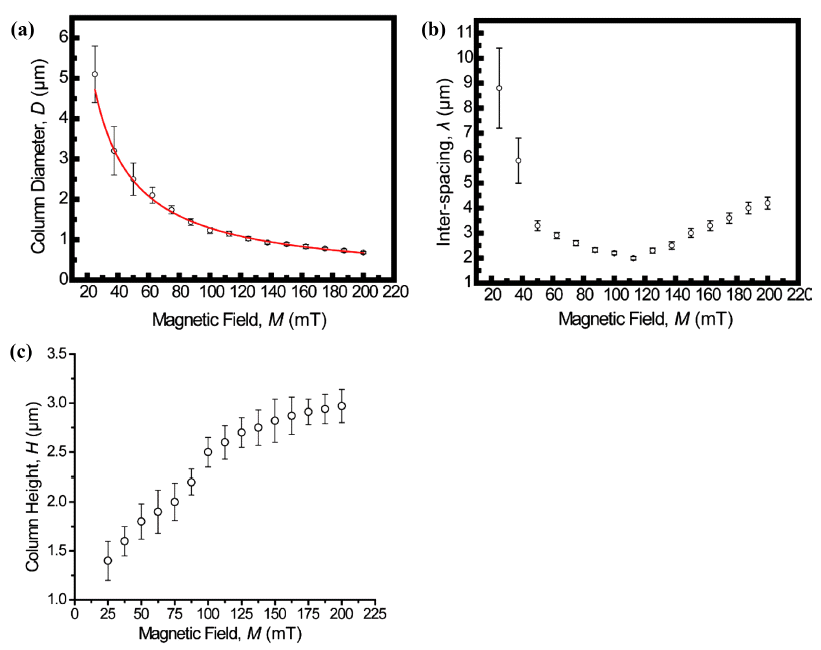
Dimensional features of vertically packed columns with diameter of 5~0.7 μm (A), interparticle spacing of 9~2 μm (B), and height of 1.3~3 μm (C).

- We experimentally observed ferrofluidic assembly and numerically analyzed the resulting lattice structures. Understanding of assembly mechanism of ordered nanoparticles aggregations can be made by competition between rotational force of the single domain particle defined as Brownian motion and magnetization vector rotation. This study shed light on extended patterning research via combined techniques of magnetic field and controlled evaporative selfassembly.
Conclusions
-
Acknowledgements
- This research work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2015R1C1A1A01053111).
Acknowledgement
- 1. R.E. Rosenweig, M. Zahn and R. Shumovich: J. Magn. Magn. Mater., (1983) 39 127.
- 2. A.J. Dickstein, S. Erramilli, R.E. Goldstein, D.P. Jackson and S.A. Langer: Science., (1993) 261 1012.Article
- 3. M. Seul and D. Andelman: Science., (1993) 267 1012.
- 4. N. E. Voicu, S. Harkema and U. Steiner: Adv. Funct. Mater.., (2006) 16 926.Article
- 5. M.D. Cowley and R.E. Rosensweig: J. Fluid Mech., (1967) 30 671.Article
- 6. C.Y. Hong, I.J. Jang, H.E. Horng, C.J. Hsu, Y.D. Yao and H.C. Yang: J. Appl. Phys., (1997) 81 4275.ArticlePDF
- 7. F. Elias, C. Flament, J.C. Bacri and S. Neveu: J. Phys. I., (1997) 7 711.Article
- 8. C.Y. Hong, C.H. Lin, C.H. Chen, Y.P. Chiu, S.Y. Yang, H.E. Horng and H.C. Yang: J. Magn. Magn. Mater., (2001) 1881.
- 9. J. Richardi, D. Ingert and M.P. Pileni: Phys. Rev. E., (2002) 66 046306.
- 10. L. Carrillo, J. Soriano and J. Ortin: Phys. Fluids., (2000) 12 1685.ArticlePDF
- 11. C.Y. Chen, Y.S. Yang and J.A. Miranda: Phys. Rev. E., (2009) 80 016314.
- 12. H.E. Horng, C.Y. Hong, W.B. Yeung and H.C. Yang: Appl. Opt., (1998) 37 2674.Article
- 13. S.H. Sun, H. Zeng, D.B. Robinson, S. Raoux, P.M. Rice, S.X. Wang and G.X. Li: J. Am. Chem. Soc., (2004) 126 273.Article
- 14. J. Legrand, A. Ngo, C. Petit and M.P. Pileni: Adv. Mater., (2001) 13 58.Article
- 15. V. Germain, J. Richardi, D. Ingert and M.P. Pileni: J. Phys. Chem. B., (2005) 109 5541.Article
Figure & Data
References
Citations
Citations to this article as recorded by 

Magnetically Driven Assemblies of γ-Fe3O4 Nanoparticles into Well-Ordered Permanent Structures
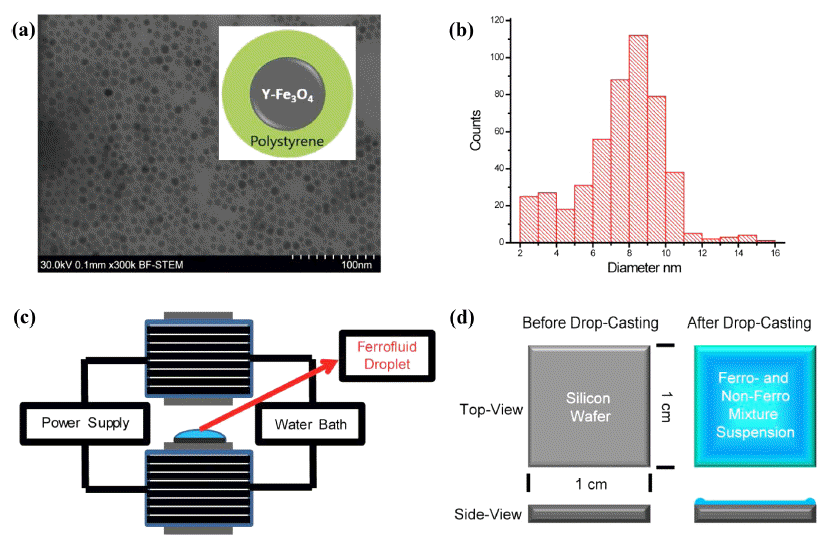
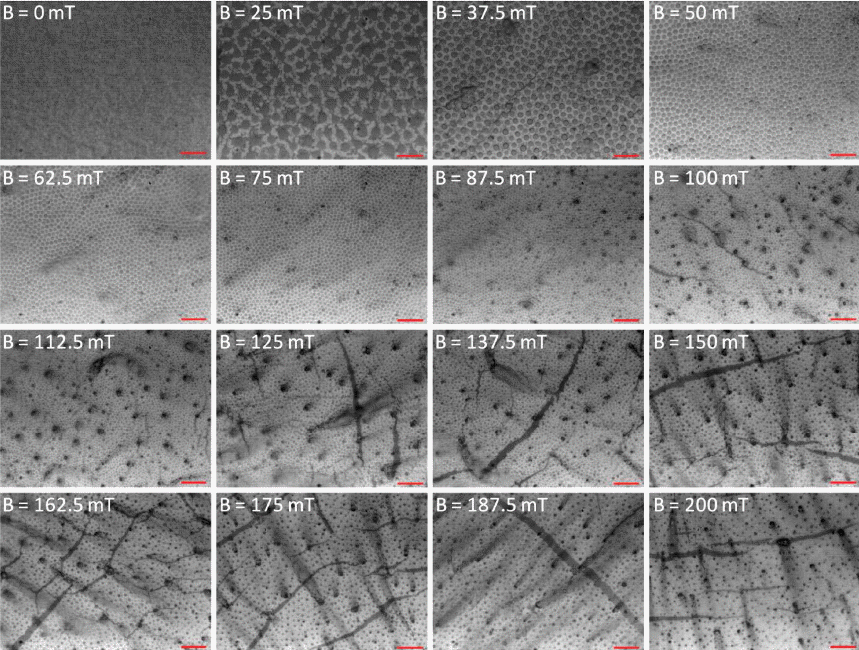
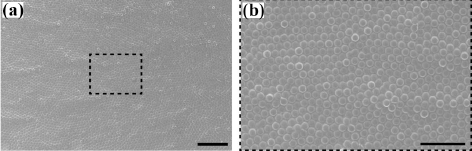
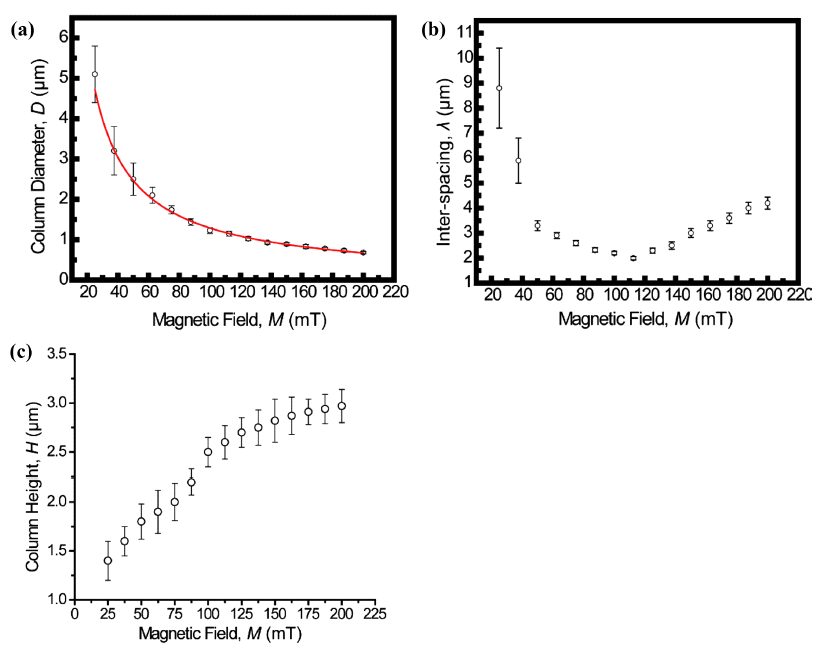
Fig. 1
(A) TEM image of the chemically synthesized γ-Fe3O4 nanoparticles. Inset is a schematic cartoon for the γ-Fe3O4 nanoparticle capped with low molecular weight polystyrene. (B) Size distribution of the nanoparticles. (C) A schematic illustration of the externally applied magnetic field during drying process of the unbound sessile single droplet of a mixture of a ferrofluid (FF) and nonmagnetic fluid (NF). (D) A schematic illustration of the unbound sessile single droplet of a mixture of a ferrofluid (FF) and nonmagnetic fluid (NF) before and after drop-casting.
Fig. 2
Morphological evolution of the magnetically driven γ-Fe3O4 nanoparticles aggregations with varying magnetic field intensity. Changes of the lateral width and interspacing of the nanoparticle aggregations with varying magnetic field intensity. All scale bars are 10 μm.
Fig. 3
High resolution FESEM images showing the assembled columns composed of close-packed the γ-Fe3O4 nanoparticles aggregations. The scale bars are 20 μm for A and 10 μm for B, respectively.
Fig. 4
Dimensional features of vertically packed columns with diameter of 5~0.7 μm (A), interparticle spacing of 9~2 μm (B), and height of 1.3~3 μm (C).
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Magnetically Driven Assemblies of γ-Fe3O4 Nanoparticles into Well-Ordered Permanent Structures
TOP
 KPMI
KPMI

 Cite this Article
Cite this Article




