Articles
- Page Path
- HOME > J Korean Powder Metall Inst > Volume 24(4); 2017 > Article
-
ARTICLE
- Expansion of Multi-wall Carbon Nanotubes and its Lithium Storage Property
- Jung-Ho Ahn*, Jeong-Seok Ahn
-
Journal of Korean Powder Metallurgy Institute 2017;24(4):275-278.
DOI: https://doi.org/10.4150/KPMI.2017.24.4.275
Published online: July 31, 2017
School of Advanced Materials Engineering, Andong National University, 1375 Gyeongdong-ro, Andong, Gyungbuk 36729, Republic of Korea
- *Corresponding Author: Jung-Ho Ahn, +82-54-820-5648, +82-54-6126, jhahn@anu.ac.kr
• Received: July 12, 2017 • Revised: August 8, 2017 • Accepted: August 10, 2017
© The Korean Powder Metallurgy Institute. All rights reserved.
- 766 Views
- 2 Download
Abstract
- In the present work, we apply a technique that has been used for the expansion of graphite to multiwall carbon nanotubes (MWCNT). The nanotubes are rapidly heated for a short duration, followed by immersion in acid solution, so that they undergo expansion. The diameter of the expanded CNTs is 5-10 times larger than that of the asreceived nanotubes. This results in considerable swelling of the CNTs and opening of the tube tips, which may facilitate the accessibility of lithium ions into the inner holes and the interstices between the nanotube walls. The Li-ion storage capacity of the expanded nanotubes is measured by using the material as an anode in Li-ion cells. The result show that the discharge capacity of the expanded nanotubes in the first cycle is as high as 2,160 mAh/g, which is about 28% higher than that of the un-treated MWCNT anode. However, the charge/discharge capacity quickly drops in subsequent cycles and finally reaches equilibrium values of ~370 mAh/g. This is possibly due to the destruction of the lattice structures by repeated intercalation of Li ions.
- Since its discovery, carbon nanotube (CNT) has been attracted much attention for energy-related applications such as hydrogen storage and electrode materials for Liion battery. Initially, it has been thought that a large amount of hydrogen atoms or lithium ions can be intercalated into the crystalline sites of CNTs such as the inner hole and the interstice between tube walls in the case of multi-walled carbon nanotubes (MWCNTs). However, the reported energy storage properties of CNTs are so far unsatisfactory [1]. For instance, as an anode material in Li-ion batteries, the charge/discharge capacity of CNTs has been reported to be quite high at the initial stage, but quickly degrades upon repeated cycling. One of the causes is the destruction of lattice structures by repeated intercalation of lithium ions. As well, the lack of suitable crystalline sites for lithium ions might be attributed to the insufficient storage property. For conventionally synthesized CNTs, their tips are usually closed, and the interwall interstices are blocked or partitioned, hindering a reversible insertion of lithium ions.
- Graphite, which is currently used as anode material for Li-ion batteries having the same chemical composition as CNTs, exhibits a theoretical capacity of 372 mAh/g. For graphite, some structurally modified forms have been widely used for many practical uses. For instance, ‘expanded graphite’ which are fabricated by a combined processing of chemical and thermal treatments, have been successfully used as an insulation foam, coating materials, additives for textile and rubber [2]. In the case of expanded graphite, the interstice distance between graphite layers can be enlarged by chemico-thermal treatments. According to literature survey, however, the expansion of MWCNTs has not been reported yet, although several research groups have investigated the structural modification of CNTs employing a variety of techniques such as ballmilling, acid treatment, etc. [3, 4, 5, 6].
- In the present work, we have attempted for the first time the technique which has been used for the synthesis of expanded graphite, to MWCNTs. The synthesized MWCNTs with structural modification were examined as an anode material for Li-ion cells. What we expected from the expanded MWCNTs was the enlargement of inter-wall distance, the destruction of wall partitions as well as the opening of nanotubes’ tip to facilitate the free and reversible intercalation of lithium ions.
Introduction
- The starting materials were MWCNTs which were synthesized by chemical vapor deposition (CVD). MWCNTs were put in an alumina crucible and then placed in a ultra-red radiation heating furnace for thermal expansion of MWCNTs. The samples were heated to 1050°C within 1 min and kept for 15 seconds in air, followed by rapid cooling to ambient temperature. 5 grams of the heat-treated MWCNTs and 200 ml of sulphuric acid (98%, Merck Co.) were mixed and stirred in a flask. While stirring, 5 grams of KMnO4 was added to the mixture for 24 h at 30°C. Then, 200 mL of de-ionized water and 50 mL of H2O2 were slowly poured into the mixture and stirred for 30 min. The expanded MWCNTs in the solution were collected by centrifugal separation. The products were washed with de-ionized water until the pH of solution reached to about 6~7. The samples were then dried at 110°C for 12 h in air. The morphology of MWCNTs were examined by a field-emission SEM (JSM-6700F) and a TEM (JEM-2010). For Li-ion cell preparation, the active material (MWCNTs), carbon black and PVDF (polyvinylidene fluoride) were mixed with a ratio of 8:1:1 to make a slurry. Before mixing, 5 g of PVDF is diluted with 5 mL of N-Methyl-2-pyrrolidinone (CH3NC4H6O). The slurry was coated on Cu foil with the thickness of 100~150 μm. The prepared electrode was assembled in a coin cell with electrolyte (LiPF6, ethylene carbonate [EC]/diethyl carbonate [DEC] 1:1 vol., Merck Co) and separator (Celgard 2300). The lithium was used as a counter and reference electrode. The cells are charged and discharged between 0.001 to 2.5 V. Charging and discharging current is 0.2 mAh/g.
Experiments
- FE-SEM morphology of as-synthesized (un-treated) and expanded MWCNTs are compared in Fig. 1. Although the diameter of the as-synthesized MWCNTs varies with individual tubes (Fig. 1a), it does not exceed 20 nm. After the expansion treatment, however, the diameter of MWCNTs increased up to ~70 nm, about 5 times larger than initial tubes (Fig. 1b). It can be also seen that some nanotubes remained intact even after the expansion treatment.
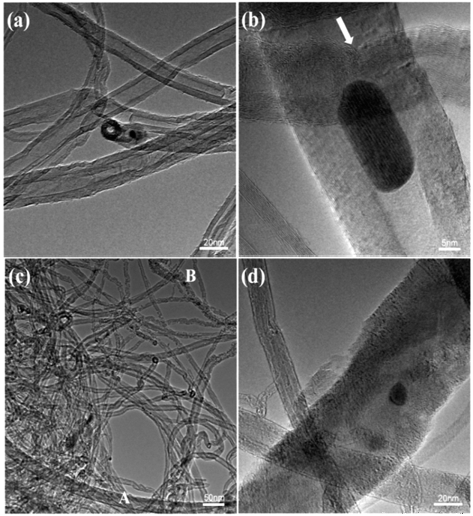
- More detailed structures were examined by TEM. The examined morphology of as-synthesized (un-treated) MWCNTs is shown in Figs. 2 (a and b). As shown in the photos, the as-synthesized MWCNTs have a diameter of ~20 nm with closed tips (Fig. 1a). At a higher magnification (Fig. 1b), a partition of tube walls (indicated as an arrow in the photo) are clearly visible as well as a Co particle which is embedded inside the nanotube. The Co has been initially used as a catalytic metal for the CVD synthesis of nanotubes. The catalystic Co particles usually remained as residues. We thought that the observed structure of as-synthesized MWCNTs with residue nanoparti- cles, wall-partitions and closed tips will hinder an accessibility of lithium ions into nanotubes. For the expanded MWCNTs which were processed in the present work (Figs. 1c and d), however, the diameter of some nanotubes increased to ~70 nm (‘A’ in the photo). The rest of the nanotubes in the photo, on the other hand, did not expand by the present chemico-thermal treatment, while some of them collapsed to amorphous structures with irregular surfaces (‘B’ in the photo). The volume fraction of expanded MWCNTs were approximately estimated by FE-SEM and TEM, and it was 30~40%.
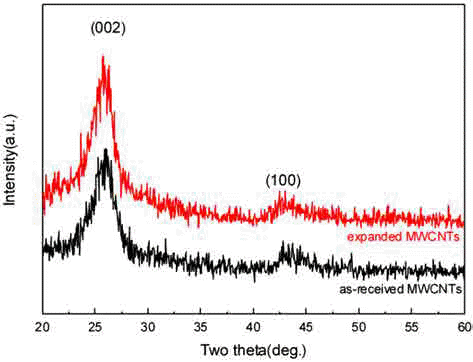
- No apparent difference in XRD patterns was observed between as-synthesized and expanded MWCNTs. This is due to the fact that 60~70 vol.% of intact MWCNTs persisted in the sample after the expansion treatment, and that aligned structures retained in expanded MWCNTs.
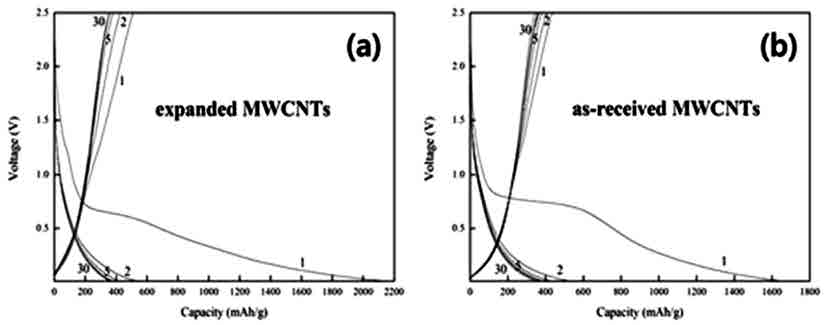
- The Li-storage properties of the expanded MWCNTs were compared with that of un-treated ones by using the materials as an anode in Li-ion cells. Fig. 4(a) displays the charge/discharge curves for the expanded MWCNT anode. The discharge capacity at the first cycle is as high as 2,160 mAh/g, demonstrating that a large amount of lithium ions were inserted in the expanded MWCNTs. However, the charge capacity at the first cycle dropped to 508 mAh/g. This indicates that about 75% of lithium ions intercalated during the first discharge were not reversibly extracted from the expanded MWCNTs. After the second cycle, the charge/discharge capacities gradually decreased and reached an equilibrium value of ~374 mAh/g after the tenth cycle. For comparison, the charge/discharge behavior of as-received (un-treated) MWCNT anode was examined (Fig. 4b). Its discharge capacity at the first cycle was 1,690 mAh/g, about 21% lower than that of the expanded MWCNT anode. Here again, the charge capacity value dropped to 434 mAh/g during the first cycle. The equilibrium capacity value after the tenth cycle was 374 mAh/g, similar to that of the expanded MWCNT anode.
- As shown in the graphs, the initial discharge values of both as-received and expanded MWCNT anodes (1,690 and 2,160 mAh/g, respectively) are much higher than the theoretical capacity of currently using graphite anode (372 mAh/g). It is though that the observed increase in initial discharge Is related to increase in the specific area, which was produced by the expansion of nanotubes. In particular, the expanded MWCNT exhibited a much higher capacity than the as-synthesized one, indicating that lithium ions can be more abundantly and freely inserted into the nanotubes. It is thus clear that the expansion of MWCNTs was effective for providing more accessible sites for lithium intercalation. However, the capacity efficiency during the first cycle was only 25%, and the reversible (equilibrium) capacity after the tenth cycle was almost identical with that of graphite. It is supposed that this might be due to the destruction of nanotube structures by lithium ions by, for instance, forming chemically stable compounds.
Results and discussion
Fig. 1
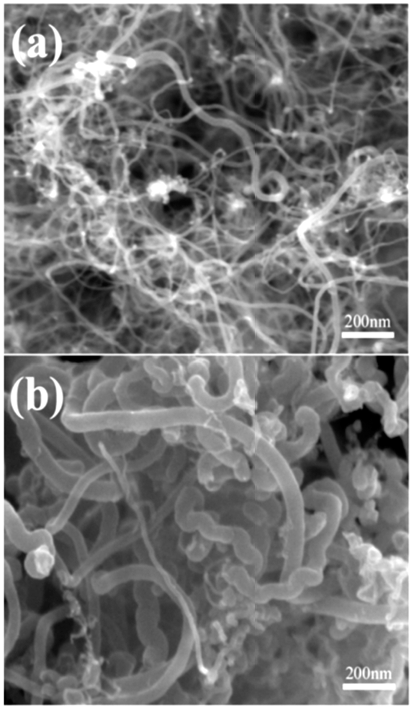
FE-SEM images of MWCNTs: (a) as-received MWCNTs, (b) expanded MWCNTs. (the same of magnification).

- In summary, we applied the expansion technique which has been traditionally used for graphite, to multi-wall carbon tubes. We expected that expanded nanotubes may facilitate the accessibility of lithium ions into the inner holes and the interstices between walls. In fact, the result of Li-ion cell tests showed that the discharge capacity of the expanded MWCNTs at the first cycle is much higher than the un-treated MWCNTs. Provided that the present method was not optimized to obtain fully expanded MWCNTs, further improvement is possible. However, the problem of low capacity efficiency after the first cycle is a major challenge to overcome for our expanded MWCNTs.
Conclusions
-
Acknowledgements
- This work was supported by a grant from 2015 Research Funds of Andong National University.
Acknowledgement
- 1. S.R. Mukai, T. Hasegawa, M. Takagi and H. Tamon: Carbon., (2004) 42 837.Article
- 2. S. Stankovich, D.A. Dikin, R.D. Piner, K.A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S.T. Nguyen and R.S. Ruoff: Carbon., (2007) 45 1558.Article
- 3. Y.B. Li, B.Q. Wei, J. Liang, Q. Yu and D.H. Wu: Carbon., (1999) 37 493.Article
- 4. Y. Lu: Adv. Mat. Res., (2012) 499 72.Article
- 5. Ph. Mauron, Ch. Emmenegger, A. Züttel, Ch. Nützenadel, P. Sudan and L. Schlapbach: Carbon., (2002) 40 1339.Article
- 6. H.R. Nam and J.H. Ahn: J. Koren Powder Metall. Inst., (2013) 20 258.Article
Figure & Data
References
Citations
Citations to this article as recorded by 

Expansion of Multi-wall Carbon Nanotubes and its Lithium Storage Property
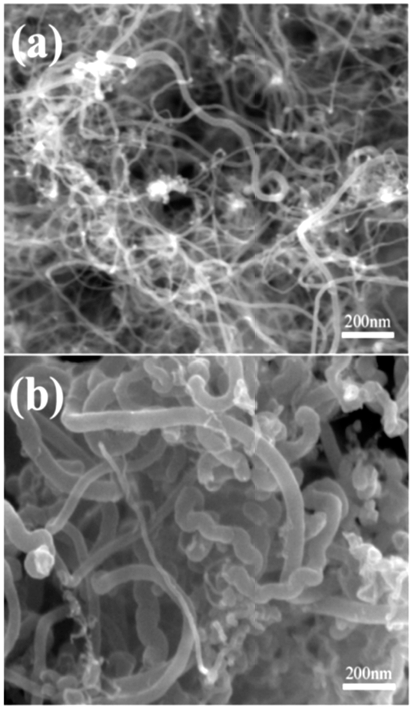
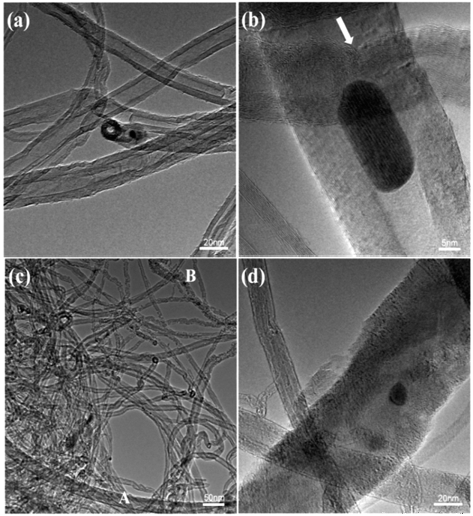
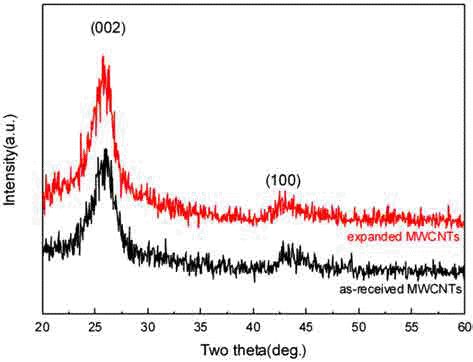
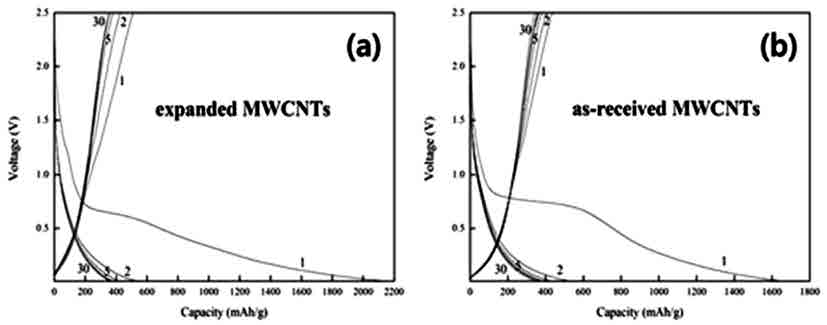
Fig. 1
FE-SEM images of MWCNTs: (a) as-received MWCNTs, (b) expanded MWCNTs. (the same of magnification).
Fig. 2
TEM images of MWCNTs: (a) and (b) as-received MWCNTs, (c) and (d) expanded MWCNTs.
Fig. 3
XRD patterns of as-received and expanded MWCNTs.
Fig. 4
Discharge-charge curves of as-received MWCNT anode in Li-ion cells.
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Expansion of Multi-wall Carbon Nanotubes and its Lithium Storage Property
TOP
 KPMI
KPMI

 Cite this Article
Cite this Article




