Articles
- Page Path
- HOME > J Korean Powder Metall Inst > Volume 24(5); 2017 > Article
-
ARTICLE
- The Synthesis Method of Tin Dioxide Nanoparticles by Plasma-Assisted Electrolysis Process and Gas Sensing Property
- Tae Hyung Kim, Yoseb Songa, Chan-Gi Lee*, Yong-Ho Choaa,*
-
Journal of Korean Powder Metallurgy Institute 2017;24(5):351-356.
DOI: https://doi.org/10.4150/KPMI.2017.24.5.351
Published online: September 30, 2017
Advanced Materials & Processing Center, Institute for Advanced Engineering (IAE), Yongin 17180, Republic of Korea
a Department of Fusion Chemical Engineering, Hanyang University, Ansan 15588, Republic of Korea
-
*Corresponding Author: Yong-Ho Choa, +82-31-400-5650, +82-31-418-6490, choa15@hanyang.ac.kr
*Co Corresponding author : Chan Gi Lee, +82-31-330-7495, +82-31-330-7116, cglee@iae.re.kr
• Received: October 16, 2017 • Revised: October 21, 2017 • Accepted: October 23, 2017
© The Korean Powder Metallurgy Institute. All rights reserved.
- 1,031 Views
- 1 Download
- 1 Crossref
Figure & Data
References
Citations
Citations to this article as recorded by 

- Effects of porosity and particle size on the gas sensing properties of SnO2 films
Min Ah Han, Hyun-Jong Kim, Hee Chul Lee, Jin-Seong Park, Ho-Nyun Lee
Applied Surface Science.2019; 481: 133. CrossRef
The Synthesis Method of Tin Dioxide Nanoparticles by Plasma-Assisted Electrolysis Process and Gas Sensing Property
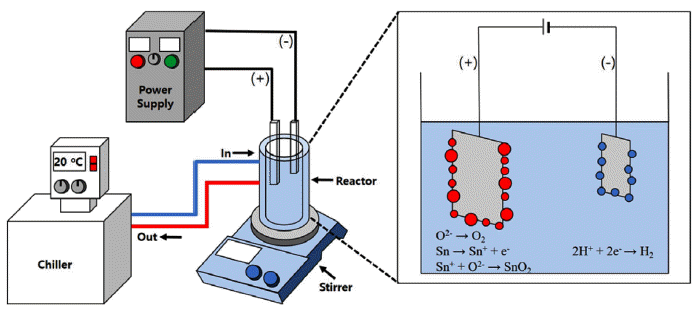
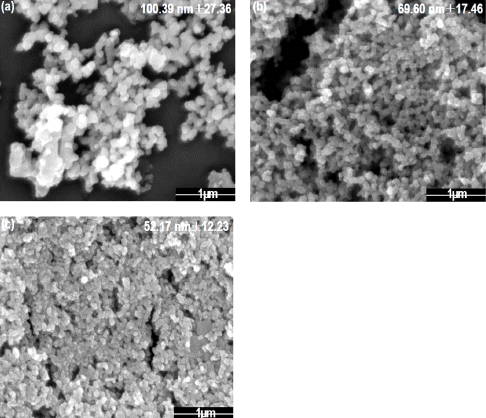
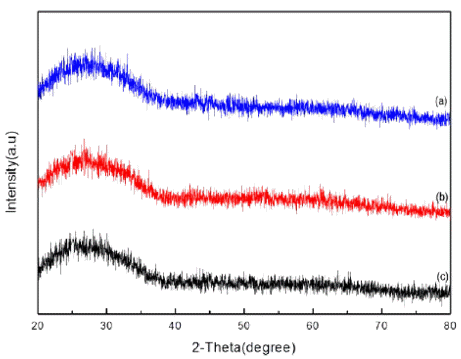
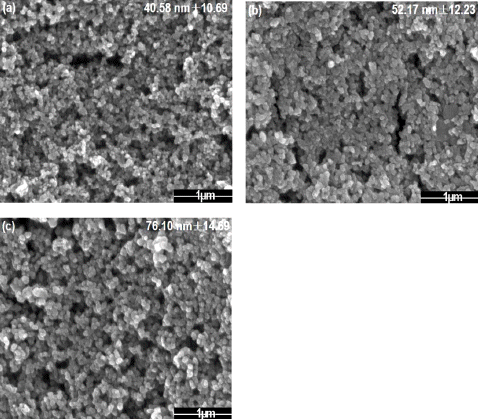
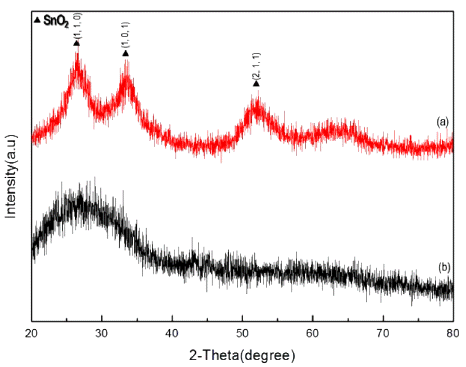
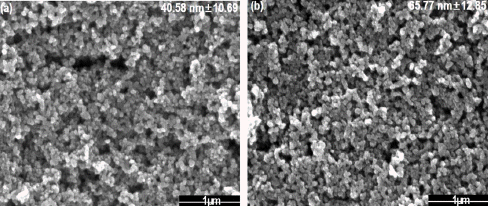
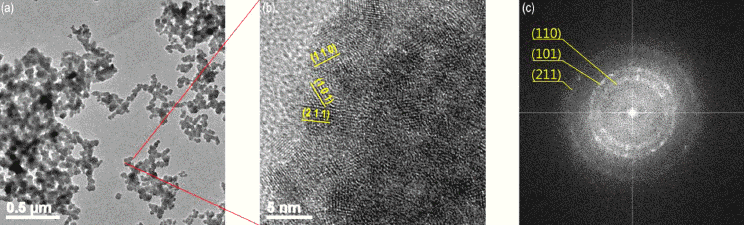
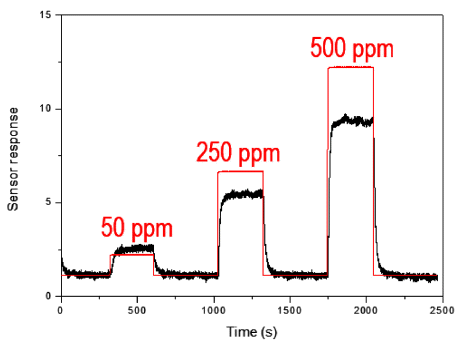
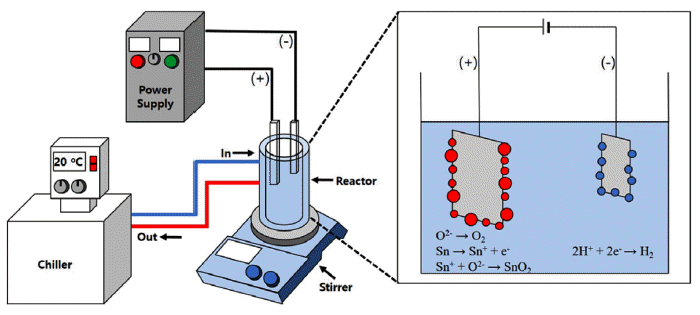
Fig. 1
Schematic diagram of plasma-assisted electrolysis process.
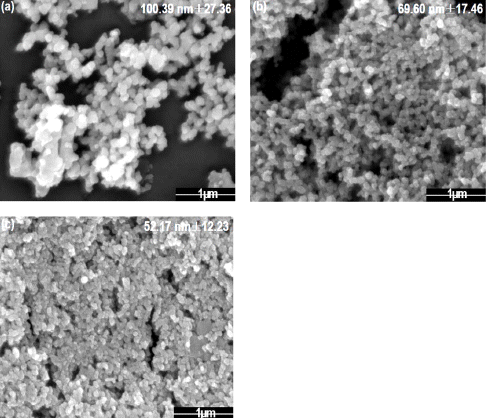
Fig. 2
FE-SEM images of tin dioxide nanoparticle synthesized by plasma-assisted electrolysis process with sulfuric acid electrolyte, (a) 5mM, (b) 7 mM, and (c) 10mM.
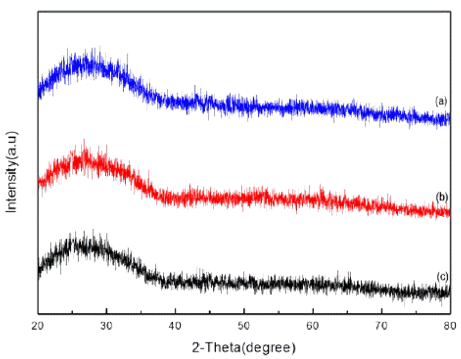
Fig. 3
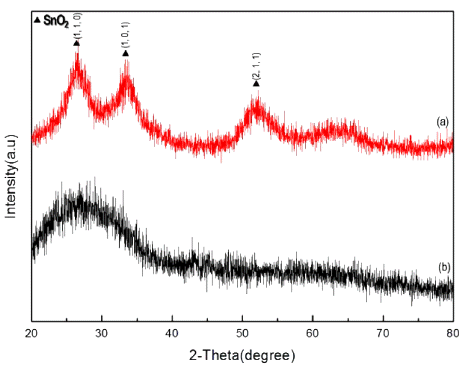
XRD patterns of tin dioxide nanoparticle by changing the mole of electrolyte (a) 5 mM (b) 7 mM (c) 10 mM.
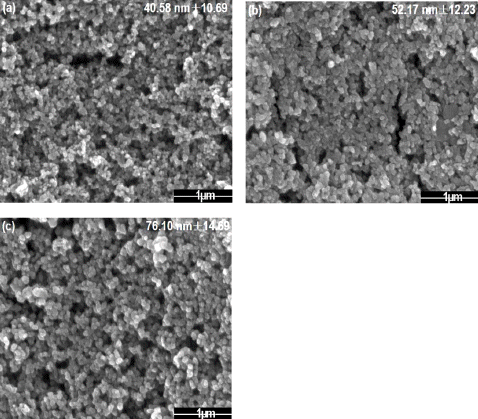
Fig. 4
FE-SEM images of tin dioxide nanoparticle by changing the frequency (a) 50Hz (b) 100Hz (c) 200Hz.
Fig. 5
XRD patterns of nanoparticle (a) after calcination at 600°C and (b) before calcination.
Fig. 6
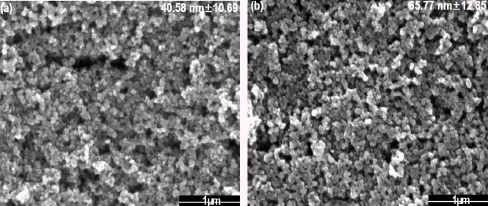
FE-SEM images of synthesized nanoparticle calcinated at 10 mM sulfuric acid (a) before calcination and (b) after calcination at 600°C.
Fig. 7
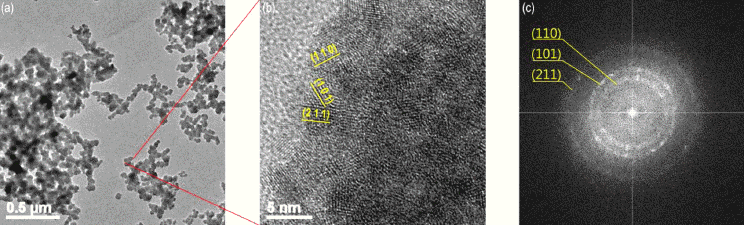
TEM images of tin dioxide powder (a) as-prepared nanoparticle at low resolution (b) at high resolution (c) FFT pattern.
Fig. 8
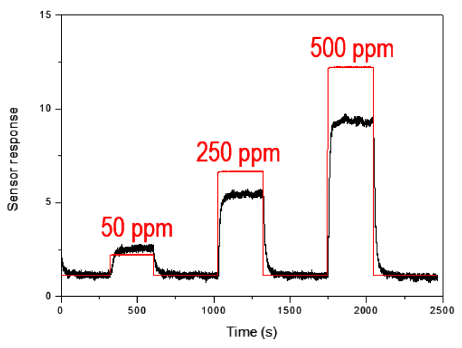
Sensing properties of as-prepared tin dioxide gas sensor with sensor response and gas flow rate.
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
Fig. 6
Fig. 7
Fig. 8
The Synthesis Method of Tin Dioxide Nanoparticles by Plasma-Assisted Electrolysis Process and Gas Sensing Property
TOP
 KPMI
KPMI

 Cite this Article
Cite this Article








