Articles
- Page Path
- HOME > J Korean Powder Metall Inst > Volume 25(2); 2018 > Article
-
ARTICLE
- Solid-state sintering mechanism of blended elemental Ti-6Al-4V powders
- Youngmoo Kim*, Young-Beom Song, Sung Ho Lee
-
Journal of Korean Powder Metallurgy Institute 2018;25(2):109-119.
DOI: https://doi.org/10.4150/KPMI.2017.25.2.109
Published online: March 31, 2018
Agency for Defense Development, P.O. Box 35, Yuseong-gu, Daejeon 34186, Republic of Korea
- *Corresponding Author: Youngmoo Kim, TEL: +82-42-821-2909, E-mail: youngmookim78@gmail.com
• Received: March 26, 2018 • Revised: March 29, 2018 • Accepted: April 13, 2018
© The Korean Powder Metallurgy Institute. All rights reserved.
- 870 Views
- 8 Download
Abstract
- The objective of this study is to reveal the sintering mechanism of mixed Ti-6Al-4V powders considering the densification and the homogenization between Ti and Al/V particles. It is found that the addition of master alloy particles into Ti enhances densification by the migration of Al into the Ti matrix prior to the self-diffusion of Ti. However, as Ti particles become coarser, sintering of the powders appears to be retarded due to slower inter-diffusion of the particles due to the reduced surface energies of Ti. Such phenomena are confirmed by a series of dilatometry tests and microstructural analyses in respect to the sintering temperature. Furthermore, the results are also consistent with the predicted activation energies for sintering. The energies are found to have decreased from 299.35 to 135.48 kJ·mol-1 by adding the Al/V particles because the activation energy for the diffusion of Al in α-Ti (77 kJ·mol-1) is much lower than that of the self-diffusion of α-Ti. The coarser Ti powders increase the energies from 135.48 to 181.16 kJ·mol-1 because the specific surface areas of Ti decrease.
- Production cost inefficiencies of wrought Ti-6Al-4V alloy are the main driving force for developing potentially lower cost processes including powder metallurgy (P/M) techniques [1-7]. The process is categorized into the blended elemental (BE) approach [8-12], prealloyed (PA) methods [13-16], additive manufacturing (AM) [17-19], spray deposition (SD) [20], metal injection molding (MIM) [21-24], far from equilibrium processing methods (rapid solidification, mechanical alloying and vapor deposition). Of these techniques, a BE route has been developed extensively owing to its feasibility and costeffectiveness in comparison to other approaches [8-12]. In general, the Ti alloy component can be produced by blending Ti, Al, and V powders, cold compaction of the mixtures followed by sintering, and if necessary, additional full-density processing of the sintered component [25]. Among these individual procedures, sintering might be largely responsible for densification of the mixed powders, and its conditions can determine the microstructural properties of the as-sintered specimens such as porosity, grain size and morphology [26]. Furthermore, since the mechanical properties, one of the most essential properties for structural applications, should be controlled by these characteristics, the performance of P/M Ti-6Al-4V components might be determined by sintering conditions.
- Solid-state sintering of mixed Ti-6Al-4V powders involves chemical concentration gradients as well as specific surface energies because there is solubility between Ti, Al, and V [12]. It is reported that for a mixture, the available surface energy is only a few J/mol, while chemical reaction energies can be thousands of times larger [27]. However, it is believed that a simple press-and-sinter process cannot deliver the full densification of mixed Ti-6Al-4V powders with homogeneous microstructure [28]. One of the reason is the use of coarse (several tens or hundreds of micrometer-sized) Ti powders for minimizing the inherent contents of impurities (C, H, N, O), resulting in decreasing the surface energy for sintering in comparison to those of ultrafine particles. In addition, since the rate at which Al and V diffuse into the Ti matrix is different, this could create Al- and V-rich regions during sintering.
- The majority of researches on sintering of Ti-6Al-4V powders have been more focused on improving the densification than achieving a uniform distribution of the alloyed elements during sintering [10, 15, 16, 29-38]. Traditionally, the increase in green density was carried out to accelerate the densification of the powders. Ivasishin demonstrated that the sintered density was obtained to 0.97 of theoretical density when the mixed Ti (-100 μm) and Al/V (-100 μm) powders were pressed at 1000 MPa [10]. The utilization of hydrogen reaction into sintering of Ti and Ti-6Al-4V powders have been studied. Hydrogen sintering and phase transformation (HSPT) processing could produce full densed sintered Ti-6Al-4V components with fine and homogeneous microstructure [36-38]. The application of intermediate TiH2 powders instead of Ti particles also enhanced the densification of the mixed powders by particle fragmentation and volume change during reduction [9, 30, 39]. The combination of applying ultra-high compaction pressure (~1000MPa) and TiH2 powders could also deliver the full density of the mixed powders [10, 29, 40]. Extremely high compaction pressure, however, might not be feasible in practical applications; for example, the production of large-scale Ti-6Al-4V components. In addition, the hydrogen embrittlement after sintering due to the lower tolerance level in the alloy and the large volume of hydrogen produced during sintering overweigh the benefits gained from the TiH2 powders. Lastly, additional full-density processing, the most widespread process, was conducted to remove residual pores in as-sintered components; for example, hot pressing and isostatic pressing, and hot forging and extrusion [13-16, 32, 34, 35, 41]. For example, several aircraft and aerospace components have been produced by a CHIP method which includes compacting and sintering followed by hot isostatic pressing [25]. Boeing Co. also developed the extrusion and rolling process of assintered Ti-6Al-4V components [32]. However, such processes might require additional facilities and labor, leading to further raising manufacturing cost. Accordingly, it is essential to introduce an alternative sintering process excluding the use of ultrahigh compacting pressure, hydrogen reaction, and further densifying processes. To develop the new consolidation technique, an in-depth investigation into the sintering mechanism of the mixed Ti-6Al-4V powders should be required considering the chemical reactions as well as the densification of particles during heating.
- However, there have been few researches on the effect of the diffusion of alloying particles on the sintering mechanism of mixed Ti-6Al-4V powders [8, 9, 12, 30, 42]. Fujita reported that Al and V particles retarded the densification of Ti particles due to the lower diffusivity of V into α-Ti matrix and Al into β-Ti one [8]. Ivasishin also revealed that the sintering mechanism of mixed TiH2-6Al-4V powders [9, 30]. The refined Ti particles by decomposition might enhance the densification of the mixtures. It is also demonstrated that the use of master alloy particles was beneficial to obtain full homogenization instead of elemental powders. Furthermore, the rate of homogenization during sintering of the mixed powders was predicted by a differential scanning calorimetry (DSC) method [42]. It is reveal that finer master alloy particles might improve the densification and homogenization of the mixed powders. However, there have been few comprehensive studies on the sintering mechanism of the mixture of Ti and Al/V particles considering physical and chemical attributes.
- Therefore, we investigated the influences of chemical concentration gradients and specific surface energies on the sintering mechanism of the mixed Ti-6Al-4V powders by investigating the densification behaviors of pure Ti particles, and a mixture of coarser Ti and Al/V powders, respectively. The behaviors of the powders were measured by a series of dilatometry tests with various sintering temperatures. The corresponding microstructures of the sintered specimens were also observed by optical and electron microscopies. The identification and quantitative analyses of phases in the sintered specimens were determined by X-ray diffraction and EDS techniques. Furthermore, the activation energies for sintering of the powders were calculated based on the data of shrinkage.
Introduction
- Three types of raw powders were used in this study: (1) commercially pure Ti (-325 mesh), (2) mixed Ti (- 325 mesh) and 60Al/40V master alloy (-325 mesh), and (3) mixed Ti (-100 mesh) and 60Al/40V master alloy (-325 mesh). The Ti and Al-V particles were produced by a hydride-dehydride (HDH) and a thermite processes, respectively. The mixtures were also fabricated by blending Ti and Al-V powders with a composition of 90 and 10 wt pct. in a Turbula mixer. These powders were designated as illustrated in Table 1. The mean particle size and its distribution of the powders were measured by a laser diffraction particle size analyzer (LS 230, Beckmann Coulter). The morphologies of BE 325, TI 100, and BE 100 were observed by a scanning electron microscopy (SEM, QUANTA 650, FEI) as shown in Fig. 1(a) to (c), respectively. The contents of the powders were measured by a spark optical emission spectrometer for Ti, Al, V, and Fe (ARL 4460, Thermo Scientific) and an inert gas fusion technique for C, H, N, and O (836 series, LECO). The compositions were confirmed to meet the standard requirement (Table II).
- The shrinkage and its rate of the powders were measured using high-resolution dilatometry (DIL 402C, Netzsche GmbH). The specimens were prepared by uniaxial compaction of the powders at 400 MPa in a cylindrical die with a diameter of 6 mm. The green densities of the compacts for BE 325, TI 325, and BE 100 were found to be 0.705, 0.712, and 0.742 of theoretical density (4.43 g/cm3) by measuring their geometries, respectively. The sample was loaded in the furnace tube with molybdenum spacers which role is to prevent the contamination from the pushrod material (Al2O3). For the same purpose, residual air in the tube was removed by subsequent flushing with high-purity Ar gas (6N) prior to heating. The specimen was then heated to the target temperature from 750 to 1350°C at a rate of 5°C/min and held for 60 min under 2.0 × 10-5 Torr. The sintered density of the sample was measured by a buoyant method based on Archimedes’ principle.
- The microstructures of the as-sintered specimens were observed by using optical microscopy (OM) and SEM (QUANTA 650, FEI). The specimens were cross-sectioned, mounted, and polished to a 0.05-μm surface finish using standard metallographic procedures. The Kroll reagent, composed of 3 mL of HF, 7 mL of HNO3, and 90 mL of distilled water, was used to etch the polished samples. The mean grain size, G, was evaluated by measuring the average linear intercept length, Gx, on a polished cross section of a sample using the following relationship [43]:
- where Gx was measured from at least 400 grains. In addition, identification and quantitative analyses of the phase in the as-sintered samples were conducted by X-ray diffraction analyses (D/MAX-2500, RIGAKU) and energy-dispersive spectrometry (EDS, QUANTA 650, FEI), respectively. Differential scanning calorimetry (DSC, LABSYS evo, SETARAM) was used to measure the reaction heat of the powder compacts during heating.
Experimental procedures
Fig. 1

Scanning electron microscopy (SEM) images showing the morphologies of (a) BE 325, (b) TI 325, and (c) BE 100.

Table 1
Designation and characterization of raw powders
- 3.1. Effect of chemical concentration gradients
- The addition of Al/V particles in Ti powders gives rise to chemical concentration gradients in the mixed Ti-6Al- 4V powders. The discrepancy in compositions brings about an additional driving force for sintering with the intent of achieving homogeneous microstructure plus the reduction of specific surface energy. Thus, in this section, to investigate the influence of the chemical concentration on the densification of the mixtures, the comparative analysis of the sintering behaviors for BE 325 and TI 325 was conducted. Figure 2 illustrates that the shrinkage and shrinkage rate of BE 325 (solid line) and TI 325 (dotted line) are given versus temperature during constant-rate heating (5°C/min), followed by holding at this temperature for 60 minutes. Above all, it is essential that the effect of green densities for both powders on the shrinkage behaviors seems to be negligible because the differences of the densities were found to be very small (1 pct.) as mentioned previously. It can be seen from Fig. 2 that the amount of shrinkage for BE 325 are larger than that of TI 325 throughout the entire temperature range. These features indicate that the added Al/V into Ti matrix enhanced the densification of Ti powders. It is also found that the onset temperatures of densification are shifted from 720 to 660°C with the existence of the mater alloy particles. It means that the sintering of the mixtures is triggered by the migration of Al into Ti, not the self-diffusion of α-Ti. The results could resolve one of the uncertain issues on the densification of mixed Ti- 6Al-4V powders in our earlier work [12]. Moreover, it is also found that the shrinkage rate of BE 325 is much higher than that of TI 325 below 900°C where α-Ti retains. On the other hand, as the temperature increased from 900 to 1020°C; i.e, Ti was transformed from α to β phases, the rate for the mixtures has slowed in comparison to that of pure Ti powders. It seems to be attributed that the phase introduced by the Al diffusion into α-Ti act as a barrier to inward and outward diffusions between V and β-Ti. Furthermore, the maximum shrinkage rate peak is observed to be shifted from 945 to 1240°C by the addition of the alloying particles. Lange demonstrated that the maximum rate could be considered as a sintering indicator [44]: below the value, the densification is dominated by densifying mechanisms, whereas above the value non-densifying mechanisms (such as grain growth) will take effect and gradually control sintering. Therefore, the addition of Al/V particles delivers that the role of densification on the whole densifying mechanism might be broadened in comparison of grain growth.
- Figure 3 shows the microstructures of the samples sintered at 850, 950, and 1050°C for BE 325 and TI 325. It can be seen from Fig. 3(a) and (b) that when the powders were sintered at 850°C, it is found that the original particles shape became disappeared and a few pores were rounded and isolated for BE 325. Otherwise, TI 325 exhibited that the shapes of particles was retained and most pores were connected. As densification progressed, in case of BE 325, the lamellar α+β colony structures and isolated pores were gradually formed. In the same manner, for TI 325, Ti grains were created and coarsened by pore structures to be closed. Especially, as the temperature approaches at 1050°C, a uniform microstructure, the colony structures, was obtained for BE 325, indicating the chemical concentration gradients might be disappeared. These microstructural characteristics were consistent with the shrinkage behaviors of the powders.
- Such tendencies are also supported by the relations of relative density and grain size versus sintering temperature for BE 325 and TI 325, as illustrated in Fig. 4. It reveals that the densities of mixed powders are higher than those of pure particles throughout the entire temperature range. The temperature where a substantial increase in the density was observed appeared to be associated with the temperature where the kink points in the shrinkage curve were found. For example, the densities of BE 325 samples are increased about 5% of theoretical density at 750 and 900°C where the slope of the shrinkage curve is changed drastically. The grain sizes for TI 325 and BE 325 were increased from 15.49 and 56.24 to 71.21 and 151.81 μm with increasing the sintering temperature, respectively. Unlike pure Ti samples, the grain sizes for BE 325 can be measured after achieving uniform microstructures above the temperature of 1050°C. It is also found that the grain coarsening of the sintered Ti sample is accelerated above 900°C. Furthermore, as shown in Fig. 2, the temperatures at the maximum shrinkage rate were 945 and 1020°C which indicates the transition of dominant densifying mechanism from densification into grain coarsening. Therefore, it can be deduced that the dominant densification mechanism of the mixtures is changed by the formation of the uniform microstructure. Otherwise, for TI 325, as Ti phase is transformed from α to β in the vicinity of 900°C, the densification might be accelerated and finally grain coarsening become more dominant. Table 2
- Lastly, to understand the effect of chemical concentration gradients on the kinetics of densification, the activation energies for sintering were predicted by the shrinkage data through the entire temperature range. Generally, the sintering kinetics of a powder can be expressed as Equation (2) [45]:
- where r is the shrinkage rate (%/min), Q is the apparent activation energy (kJ/mol), R is the universal gas constant, T is temperature (K), t is time (min), n is the time exponent independent of temperature, and C is a constant. To determine the value of ln (r), the shrinkage during sintering can be estimated by considering the coefficient of thermal expansion. The value of n can be then calculated from the slope of the linear relationship between ln (r) and ln (t). Finally, the activation energy, Q, can be estimated from the slope of the linear relationship between ln (r) and the inverse temperature. Figure 5 illustrates the experimental data of ln (r) versus 1/T (for 60 min of isothermal sintering) for BE 325 and TI 325. The data set for both powders shows a discontinuity at about 1000 and 900°C for the mixed and pure Ti powders, respectively. Therefore, activation energies for sintering were calculated for two sections which means that the dominant sintering mechanism might be changed with the temperature. The activation energies for both powders with the temperature were illustrated in Table 3. The addition of Al/V particles into Ti matrix leads to activation energies to be reduced from 299.35 and 84.45 kJ·mol-1 to 135.48 and 25.08 kJ·mol-1. According to the previous study [40], the activation energies for sintering of Ti (3-5 μm) were determined to be 184 and 92 kJ·mol-1 for α- and β-Ti, respectively. The values, 299.35 and 84.45 kJ·mol-1, in this study are found to be higher than the reported one because of coarsened Ti particle. In addition, for the mixtures, the reduced activation energies seem to be much lower energies for the diffusion of Al in α-Ti (77 kJ·mol-1) [46], which is the main densification mechanism during chemical homogenization. Consequently, this indicates that the added Al and V into Ti matrix might allow the densification of Ti particles to be more feasible by the formation of chemical concentration gradients.
- It is believed that the addition of the master alloy particles into Ti matrix might retard the densification of Ti powders by following two reasons. First, the discrepancy in solubility between Al and Ti might cause Kirkendall voids with the formation of Ti-Al intermetallic compounds around Ti particles in the early stage of sintering (650 to 850°C) [8, 9, 30]. The other is such Ti-Al phases might act as a barrier to interdiffusion between Ti and V in the β-Ti range. However, on the contrary to the previous researches, we demonstrated that the alloying elements seem to enhance the densification of Ti powders based on the following observations; the reduced onset temperature to sintering, and the higher overall dimensional change for BE 325. Especially, in the α-Ti range, the densification of the mixtures are accelerated than that of pure Ti particles. Consequently, it can be deduced that the majority of such enhancement is attributed to the Al diffusion into α-Ti matrix. Otherwise, it is also identified that, in the same manner of previous studies, a V element acts as an obstacle to sintering of the mixed Ti-6Al- 4V powders.
- 3.2. Effect of specific surface energy
- The application of coarsened Ti particle size in the mixtures was carried out to investigate the influence of the specific surface energy of particles on the densification in the mixed Ti-6Al-4V powders. It can be seen from Fig. 6 that the shrinkage and its rate behaviors of both powders appeared to be similar, and the dimensional change for BE 100 is found to be smaller than that of BE 325 throughout the entire temperature range. It is attributed that coarser Ti particles might reduce the specific surface energy for sintering, resulting in retarding the densification of the mixtures. However, the shrinkage rate of BE 325 is gradually reduced in compared to that of BE 100 above the temperature of 900°C, which seems to be related to the transformation of Ti phases and V migration. The use of coarsened Ti particles deliver the temperature for maximum shrinkage rate to be shifted from 1020 to 1035°C. As discussed previously, it indicates that the uniform microstructure of the mixed powders is achieved at slightly higher sintering temperature as Ti particle size is increased.
- These features can be confirmed by the microstructural observations as shown in Fig. 3 and 7. At the same temperature, the pore structure for BE 325 is found to be smaller and more isolated than that of BE 100. Unusually, as the samples were heated to 950°C, more lamellar α+β colony structures were found in the sintered sample for BE 100. These structures can be formed by the diffusion of V. Thus, it is concluded that the inward and outward diffusion between β-Ti and V might occur at lower temperature for coarser Ti particles because BE 100 exhibits the smaller portion of Ti-Al phases around Ti matrix which act as an obstacle to the migration of V. This seems to be consistent with the shrinkage behaviors of both mixtures above 900°C. As the temperature approaches at 1050°C, the uniform microstructures are obtained for both mixtures, indicating the chemical homogenization to be achieved.
- Figure 8 illustrates the variation of relative density and grain growth for both mixtures as illustrated in Fig. 8. The final densities of specimens for BE 325 and BE 100 are found to be 0.963 and 0.949, respectively, and the porosity for BE 325 is always lower than that of BE 325 throughout the entire temperature range. The colony size for BE 100 and BE 325 is increased from 56.24 to 151.81 μm and from 79.04 to 133.97 μm, respectively, with increasing the temperature from 1050 to 1350°C. In addition, the rate of grain coarsening with the temperature for fine particles is gradually increased than that of coarser ones. It is attributed that BE 325 exhibited higher sintered density in comparison to BE 100, resulting in lesser pinning effects on grain growth by pores.
- The influence of coarsened Ti particles on phase evolution during chemical homogenization was investigated by XRD and BSE analyses of the sintered specimens for both mixtures. It can be seen from Fig. 9(a) that for the both powders, five phases (α-Ti, β-Ti, Ti3Al, Al8V5, Al3V) involves during the homogenization process. At the beginning of densification (750°C), new phases such as Ti3Al and Al8V5 are formed with the phases of raw powders; α-Ti and Al3V. It indicates that the dissolution of the master alloy particles might trigger by chemical concentration gradients; 1) the migration of Al in Al3V to Ti matrix create Ti3Al phases around α-Ti, and 2) Aldepleted region in the Al3V phase Al transform into Al8V5. These phase transformations appear to be reasonable based on the binary phase diagrams for Ti-Al and Al-V. As the temperature increases, such intermetallic compounds are gradually disappeared. The β-Ti phase was newly formed at 950°C which is strongly related with the colony structures, and only two phases (α-and β-Ti) are detected above the temperature of 1050°C. It can be seen from Fig. 9(b) that most peaks for the finer particles are found to be broadened compared with those of coarser powders during homogenization (850°C). It means that the amount of solute elements (possibly Al and Ti) in the Ti and master alloy phases for BE 325 seems to be higher than that of BE 100. At this temperature, V appeared not to be involved in these reactions because V is insoluble with Ti below 900°C, as mentioned formerly. Such tendencies were also confirmed by BSE and EDS techniques on the specimens sintered at 850°C for both particles (Fig. 10 and Fig. 11). Regardless of Ti particle size, the microstructure can be divided into four regions, and the composition of each domain can be determined by the XRD analysis (α-Ti, Ti3Al, Al8V5, Al3V). Compared with the microstructures of both samples, several differences are found; for example, BE 325 exhibited smaller original master alloy (Al3V) phases, larger Ti3Al phases around the Ti matrix, and Aldepleted Al3V including Ti unlike BE 100 (Fig. 10(b)). As shown in Fig. 11, the small amount of Ti in the Al- depleted region, about 3.57 wt% was observed for finer Ti particles. Microstructural evolution during chemical homogenization is schematized as illustrated into Fig. 12. Consequently, it can be deduced that the interdiffusion of Al and Ti for coarser Ti particles might occur at a slower pace than that of finer Ti ones, resulting in retarding the densification of the mixtures.
- The activation energies for sintering of BE 100 and 325 were also calculated based on the Eq. (2). Figure 13 shows the experimental data of ln (r) versus 1/T (for 60 min of isothermal sintering) for BE 325 and BE 100. The slope between ln (r) versus 1/T is found to be changed drastically at about 1000°C for both powders. Thus, as the Ti particle size is coarsened, the activation energies were also increased from 135.48 and 25.08 kJ·mol-1 to 181.16 and 17.07 kJ·mol-1 for BE 325 and BE 100, respectively. The increased energies for sintering seem to be attributed to the reduced area of Ti grain boundaries which are the main source of Ti densification. Consequently, the migration of Ti might slow down resulting in the retardation of the mixed Ti-6Al-4V powders by using coarser Ti particles during chemical homogenization. Otherwise, after the termination of homogenization, the influence of grain coarsening on the densification of both mixtures appears to be similar.
Results and discussion
Fig. 2
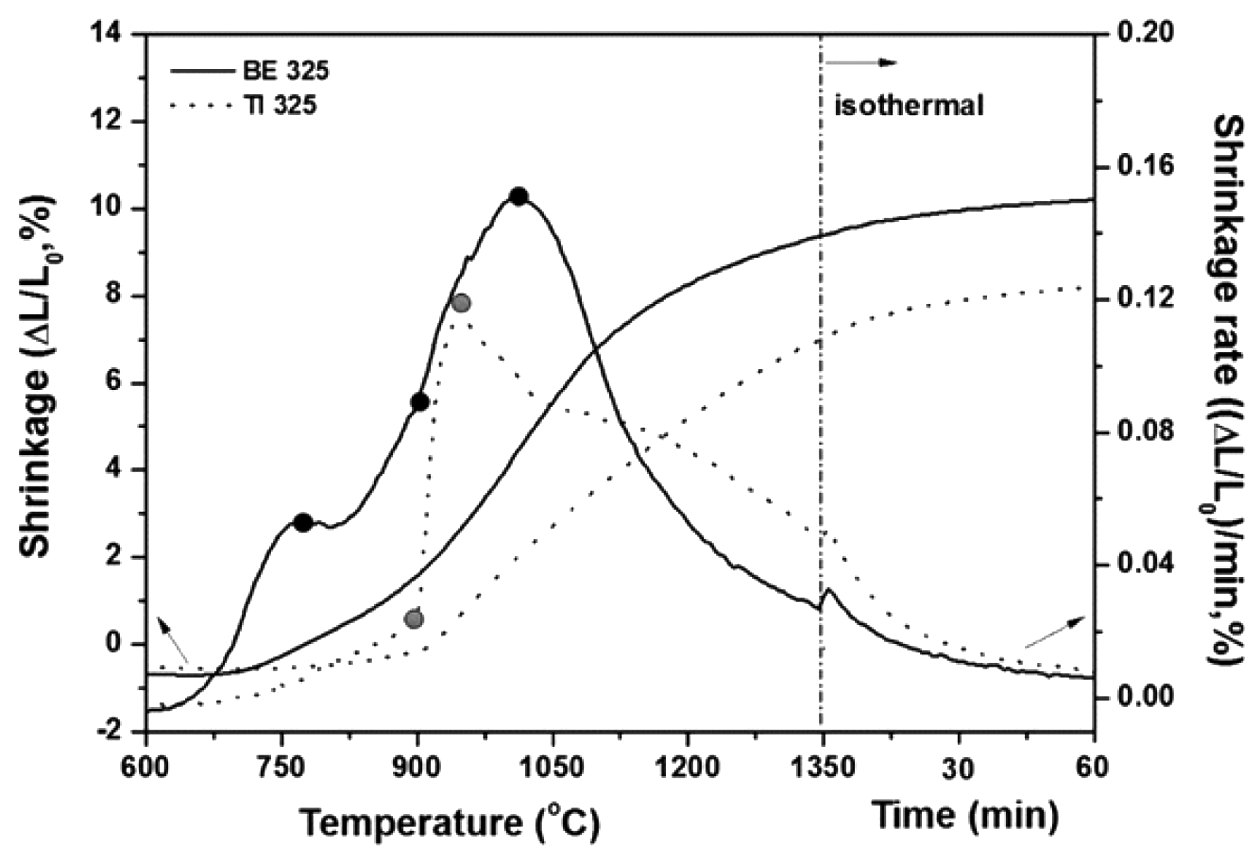
Shrinkage and shrinkage rates are given versus temperature during heating at 5°C/minutes to 1350°C, and holding at 120 minutes for BE 325 and TI 325.

Fig. 3
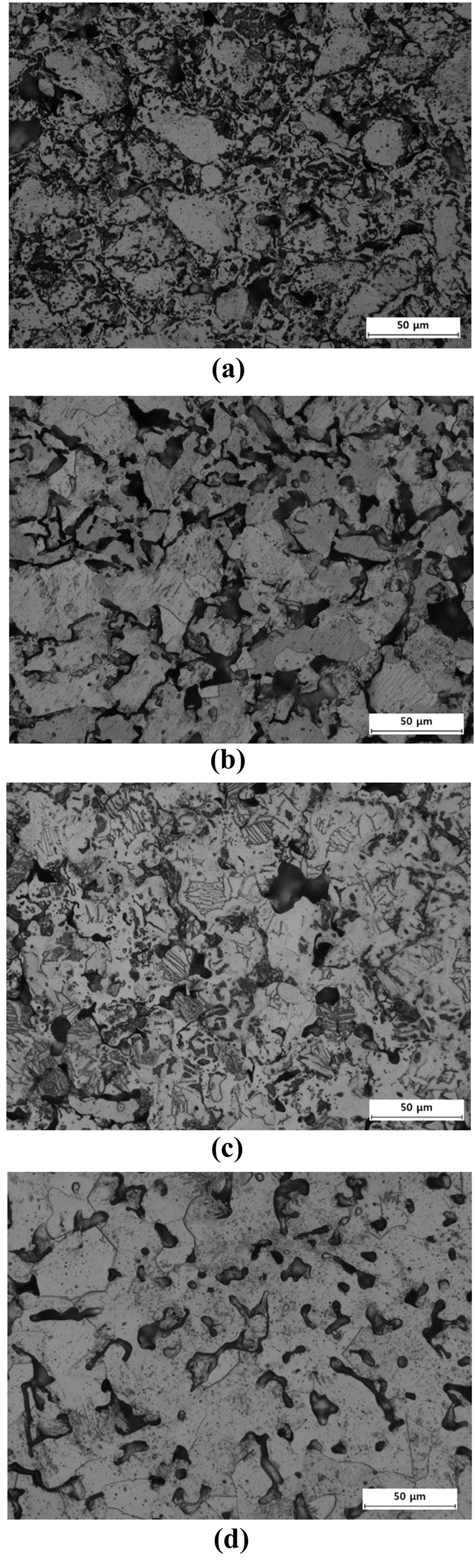
Microstructures of sintered samples for BE 325 and TI 325 heated to different temperatures of (a) and (b) 850°C, (c) and (d) 950°C, (e) and (f) 1050°C.

Fig. 4

Variations of the sintered density and average grain size of sintered samples for BE 325 and TI 325 with temperature ranging from 750 to 1350°C.

Table 2
Chemical composition of raw powders (unit: wt%)
Fig. 5
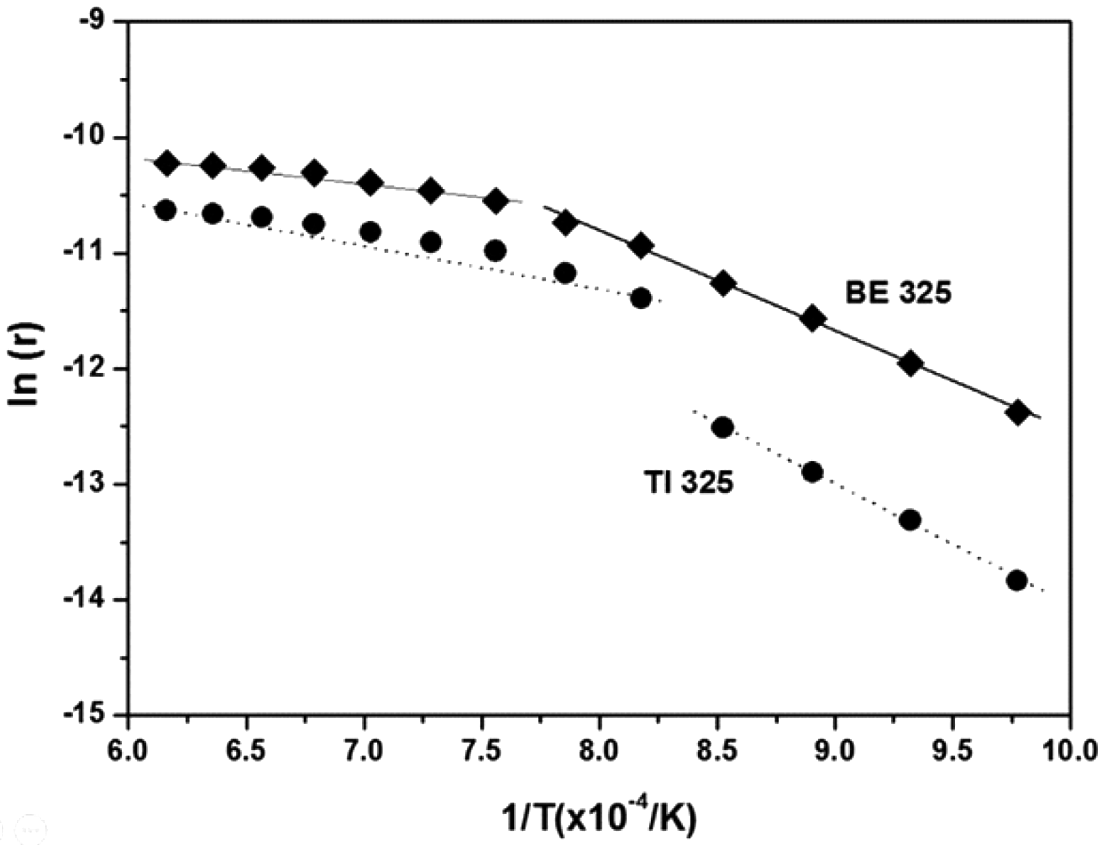
Plots of logarithmic shrinkage rate ln (r) versus 1/T to estimate the apparent activation energy for BE 325 and TI 325 compacts.

Table 3
Activation energies for sintering of raw powders with the sintering temperature
| Powder | Temperature Range (°C) | Activation Energy for Sintering (kJ/mol) |
|---|---|---|
|
|
||
| BE 325 | 750-1000 | 135.48 |
| 1050-1350 | 25.08 | |
| TI 325 | 750-900 | 299.35 |
| 950-1350 | 84.45 | |
| BE 100 | 750-1000 | 181.16 |
| 1050-1350 | 17.07 | |
Fig. 6

Shrinkage and shrinkage rates are given versus temperature during heating at 5°C/minutes to 1350°C, and holding at 120 minutes for BE 325 and BE 100.

Fig. 7
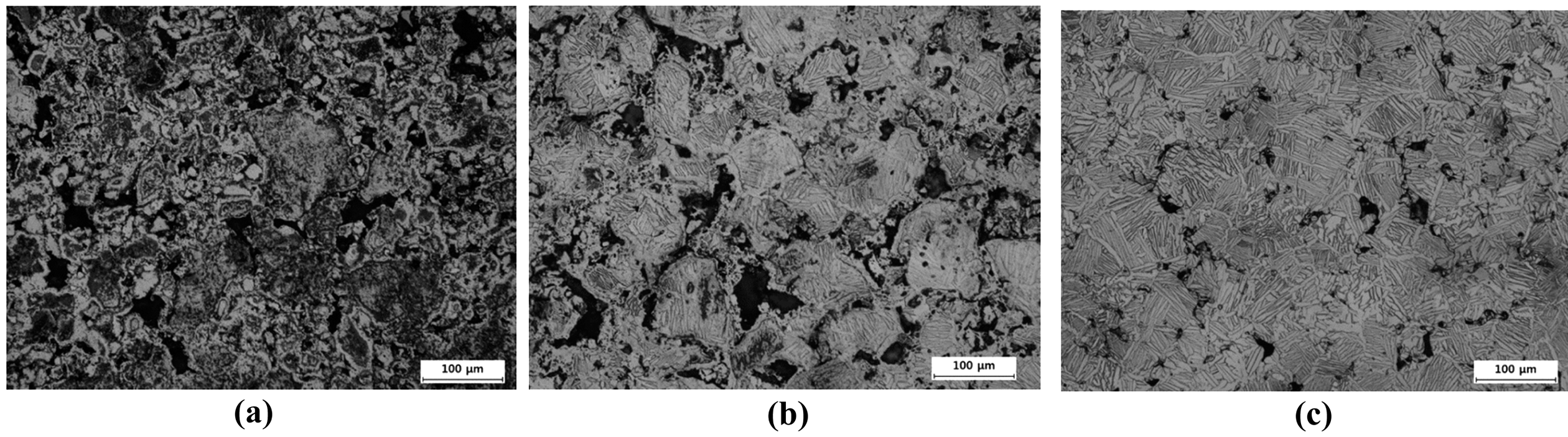
Microstructures of sintered samples for BE 100 heated to different temperatures of (a) 850°C, (b) 950°C, and (c) 1050°C.

Fig. 8

Variations of the sintered density and average grain size of sintered samples for BE 325 and BE 100 with temperature ranging from 750 to 1350°C.

Fig. 9

X-ray diffraction (XRD) patterns of the sintered Ti- 6Al-4V samples for (a) BE 325 heated to 750, 850, 950°C, and 1050°C, and (b) BE 325 and BE 100 heated to 1050°C.

Fig. 10
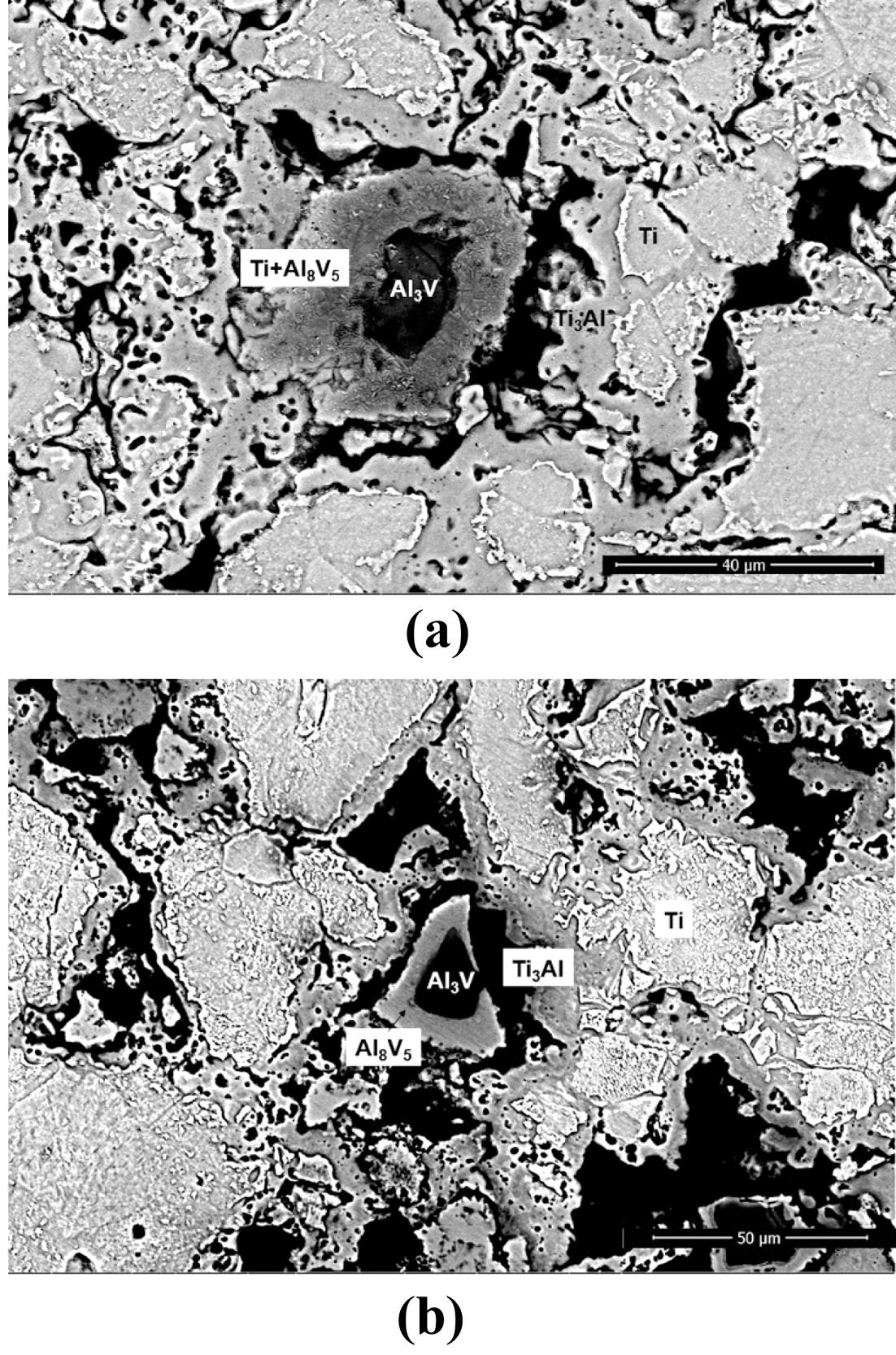
Backscattered electron images of sintered samples heated to 850°C for (a) BE 325 and (b) BE 100.

Fig. 11
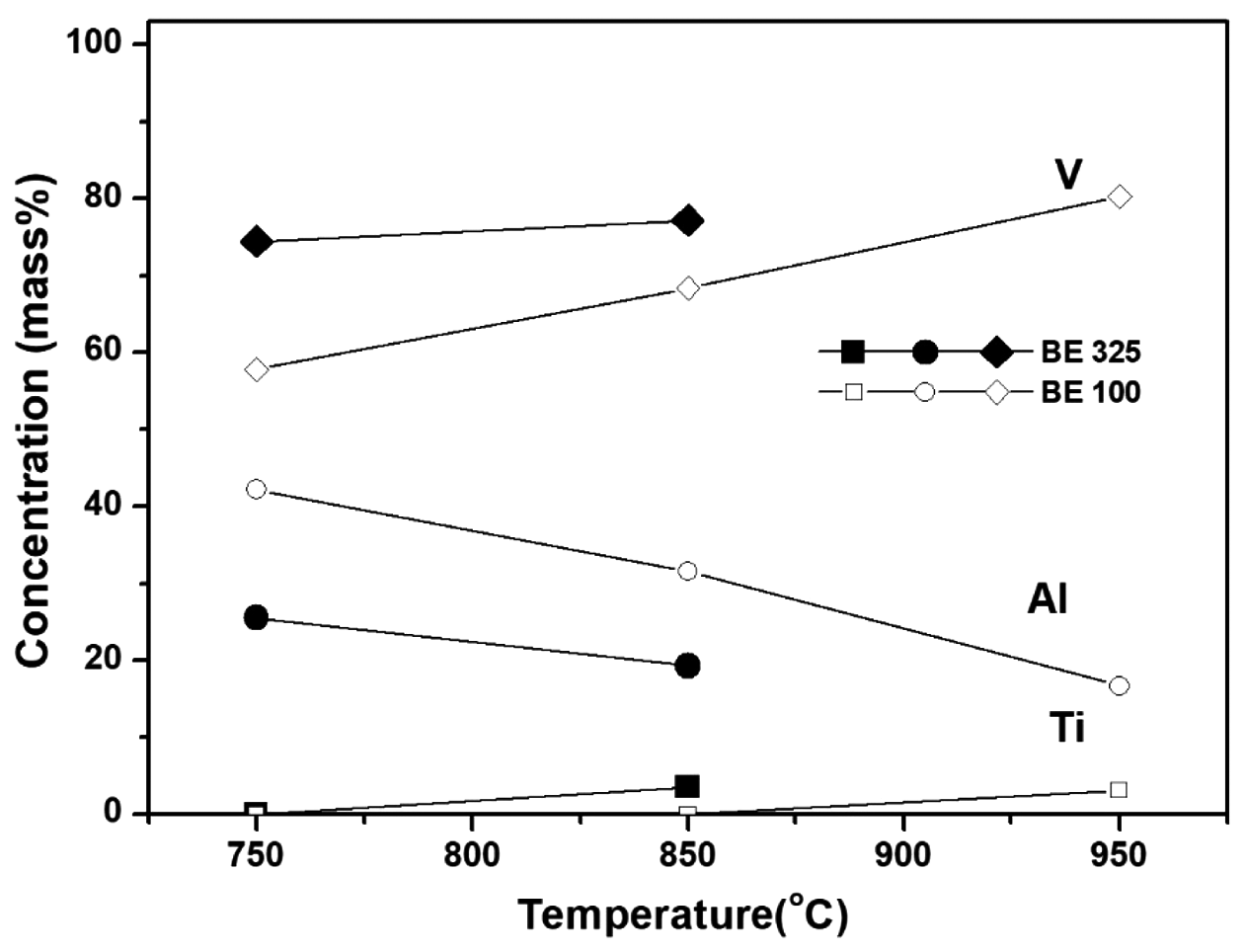
Variation in the amount of constituents with sintering temperature in the vicinity of Al/V mater alloy phase for BE 325 and BE 100.

- We investigated the influences of chemical concentration gradients as well as specific surface energies on the sintering mechanism of the mixed Ti-6Al-4V powder by evaluating the shrinkage and shrinkage rate of the powder compacts, observing the microstructures of the sintered samples, identifying the phase evolution during sintering, and predicting the apparent activation energies for densification. The major results were summarized as follows.
- Chemical concentration gradients were introduced by the addition of Al/V particles into Ti powders. Faster and further shrinkage for BE 325 was found throughout the entire temperature ranges, indicating that the densification of Ti particles was enhanced by added Al and V particles. The onset temperature to densification might be reduced to about 60°C, indicating that the sintering of the mixture triggered by the Al diffusion into Ti instead of self-diffusion of α-Ti. Furthermore, the temperature at maximum shrinkage rate peak is increased from 945 to 1240°C. It means that the role of densification on the sintering of the mixed powders to be widened versus that of grain coarsening. The relative densities for the mixed powders were found to be higher than those of Ti particles regardless of sintering temperature. Lastly, the apparent activation energies for sintering was reduced from 299.35 and 84.45 kJ/mol to 135.48 and 25.08 kJ/mol by adding the Al/V particles. Consequently, the migration of Al into Ti would be the main contributor to enhancement of the mixed Ti-6Al-4V powders.
- The influence of specific surface energies were also investigated by controlling the Ti particle size in the mixed powders. Through the dilatometry test, the final dimensional change for finer Ti particles was higher than that of coarser Ti ones. It is also found that the sintered density for BE 325 is higher than that of BE 100 throughout the entire temperature range. Through the Xray diffraction and EDS analyses, in case of finer Ti particles, the amount of solute atoms (possibly Al and Ti) in the Ti and Al-depleted master alloy phase was found to be higher compares with coarser Ti ones during chemical homogenization. Lastly, the activation energies for densification during the homogenization were increased from 135.48 to 199.94 kJ/mol for coarser Ti particles. Consequently, the reduced specific surface energies by using finer Ti particles might bring about the inter-diffusion of Ti and Al to be accelerated, resulting in enhancing the densification of the mixed Ti-6Al-4V powders.
Conclusions
-
Acknowledgements
- This research was sponsored by Dual-Use Technology Research and Development Program (Contract No. UM160309RD3) co-funded by Ministry of Trade, Industry and Energy and Defense Acquisition Program Administration.
Acknowledgement
- 1. F. Froes,, D. Eylon,, G. Eichelman, and H. Burte:: JOM., 32 (1980) 47.ArticlePDF
- 2. F. Froes, and D. Eylon:: Powder Metall. Int.., 17 (1985) 163.
- 3. V. Moxson,, O.N. Senkov, and F. Froes:: JOM., 52 (2000) 24.ArticlePDF
- 4. F. Froes,, S. Mashl,, J. Hebeisen,, V. Moxson, and V. Duz:: JOM., 56 (2004) 46.ArticlePDF
- 5. F.S. Froes,, M.N. Gungor, and M.A. Imam:: JOM., 59 (2007) 28.ArticlePDF
- 6. S. Abkowitz,, S. Abkowitz, and H. Fisher:: Met. Powder Rep.., 66 (2011) 16.
- 7. D.M. Bowden, and W.H. Peter:, Near-net shape fabrication using low-cost titanium alloy powders, in, The Boeing Company, (2012).
- 8. T. Fujita,, A. Ogawa,, C. Ouchi, and H. Tajima:: Mater. Sci. Eng. A., 213 (1996) 148.
- 9. O.M. Ivasishin,, V. Anokhin,, A. Demidik, and D.G. Savvakin:: Key Eng. Mater.., 188 (2000) 55.
- 10. O.M. Ivasishin,, D.G. Savvakin,, F.H.S. Froes, and K.A. Bondareva:: Powder Metall. Met. Ceramics., 41 (2002) 382.
- 11. L. Bolzoni,, P. Esteban,, E.M. Ruiz-Navas, and E. Gordo:: J. Mech. Behav. Biomed. Mater.., 15 (2012) 33.
- 12. Y. Kim,, J. Lee,, B. Lee,, H.J. Ryu, and S.H. Hong:: Metall. Mater. Trans., A Phys. Metall. Mater. Sci.., 47 (2016) 4616.
- 13. D. Delo, and H. Piehler:: Acta Mater.., 47 (1999) 2841.
- 14. K. Zhang,, J. Mei,, N. Wain, and X. Wu:: Metall. Mater. Trans., A Phys. Metall. Mater. Sci.., 41 (2010) 1033.
- 15. Y. Kim,, E-P. Kim,, Y-B. Song,, S.H. Lee, and Y-S. Kwon:: J. Alloys Compd.., 603 (2014) 207.
- 16. R.P. Guo,, L. Xu,, J. Wu,, Z.G. Lu, and R. Yang:: Mater. Sci. Forum., 849 (2016) 760.
- 17. A.M. Beese, and B.E. Carroll:: JOM., 68 (2016) 724.ArticlePDF
- 18. B. Dutta, and F.H. Froes:, Additive manufacturing of titanium alloys, Butterworth-Heinemann Limited, (2016).
- 19. T. Machry,, D. Eatock,, J. Meyer,, A. Antonysamy,, A. Ho, and P. Prangnell:: Powder Metall.., 59 (2016) 41.
- 20. T. Shimabukuro,, R. Daouk,, J. Skupnjak,, M. Nordman,, M. Burrell,, L. Sutanto,, A. Abad,, H. Garmestani,, N. Ula, and J. Foyos:: Defect Diffusion Forum., 367 (2016) 175.
- 21. G. Shibo,, Q. Xuanhui,, H. Xinbo,, Z. Ting, and D. Bohua:: J. Mater. Proc. Tech.., 173 (2006) 310.
- 22. O.M. Ferri,, T. Ebel, and R. Bormann:: Mater. Sci. Eng. A., 504 (2009) 107.
- 23. G.C. Obasi,, O.M. Ferri,, T. Ebel, and R. Bormann:: Mater. Sci. Eng. A., 527 (2010) 3929.
- 24. R. German:: Materials (Basel)., 6 (2013) 3641.
- 25. S. Abkowitz,, S. Abkowitz, and H. Fisher:, Titanium powder metallurgy, M. A. Qian and F. H. Froes (Ed.), Butterworth-Heinemann, Oxford, United Kingdom, (2015) 299.
- 26. H. Wang,, Z.Z. Fang, and P. Sun:: Int. J. Powder Metall.., 46 (2010) 45.
- 27. R.M. German:, Powder Metallurgy and Particulate Materials Processing, New Jersey, U.S.A, (2005) 93.
- 28. M. Qian,, Y.F. Yang,, S.D. Luo, and H.P. Tang:, Titanium powder metallurgy, M. A. Qian and F. H. Froes (Ed.), Butterworth-Heinemann, Oxford, United Kingdom, (2015) 201.
- 29. O. Ivasishin:: Mater. Sci. Forum., 624 (2005) 8.
- 30. O.M. Ivasishin,, D. Eylon,, V. Bondarchuk, and D.G. Savvakin:: Defect and Diffusion Forum., 245 (2008) 177.
- 31. D. Zhang,, S. Raynova,, V. Nadakuduru,, P. Cao,, B. Gabbitas, and B. Robinson:: Mater. Sci. Forum., 618-619 (2009) 513.
- 32. S.M. El-Soudani,, K-O. Yu,, E.M. Crist,, F. Sun,, M.B. Campbell,, T.S. Esposito,, J.J. Phillips,, V. Moxson, and V.A. Duz:: Metall. Mater. Trans., A Phys. Metall. Mater. Sci.., 44 (2013) 899.
- 33. J.D. Paramore,, Z. Zak Fang, and P. Sun:, Titanium powder metallurgy, M. A. Qian and F. H. Froes (Ed.), Butterworth-Heinemann, Oxford, United Kingdom, (2015) 163.
- 34. M. Jia, and B. Gabbitas:: Key Eng. Mater.., 704 (2016) 127.
- 35. Y. Zheng,, X. Yao , J. Liang and D. Zhang:: Metall. Mater. Trans. A., 47 (2016) 1842.ArticlePDF
- 36. P. Sun,, Z.Z. Fang, and M. Koopman:: Adv. Eng. Mater.., 15 (2013) 1007.
- 37. J.D. Paramore,, Z.Z. Fang,, P. Sun,, M. Koopman,, K.R. Chandran, and M. Dunstan:: Scr. Mater.., 107 (2015) 103.
- 38. P. Sun,, Z.Z. Fang,, M. Koopman,, Y. Xia,, J. Paramore,, K.R. Chandran,, Y. Ren, and J. Lu:: Metall. Mater. Trans., A Phys. Metall. Mater. Sci.., 46 (2015) 5546.
- 39. O. Ivasishin, and V. Moxson:, Titanium powder metallurgy, M. A. Qian and F. H. Froes (Ed.), Butterworth-Heinemann, Oxford, United Kingdom, (2015) 117.
- 40. B. Panigrahi,, M. Godkhindi,, K. Das,, P. Mukunda,, V. Dabhade, and P. Ramakrishnan:: J. Mater. Res.., 20 (2005) 827.Article
- 41. D.L. Zhang,, S. Raynova,, V. Nadakuduru,, P. Cao,, B. Gabbitas, and B. Robinson:: Mater. Sci. Forum., 315 (2009) 513.
- 42. G. Steedman, and S. Corbin:: Powder Metall.., 58 (2015) 67.
- 43. M.I. Mendelson:: J. Am. Ceram. Soc.., 52 (1969) 443.
- 44. F.F. Lange:: J. Am. Ceram. Soc.., 72 (1989) 3.
- 45. L. Pathak,, S. Mishra,, P. Mukunda,, M. Godkhindi,, D. Bhattacharya, and K. Chopra:: J. Mater. Sci.., 29 (1994) 5455.ArticlePDF
- 46. Z. Liu, and G. Welsch:: Metall. Trans., A, Phys. Metall. Mater. Sci.., 19 (1988) 1121.
Figure & Data
References
Citations
Citations to this article as recorded by 

Solid-state sintering mechanism of blended elemental Ti-6Al-4V powders


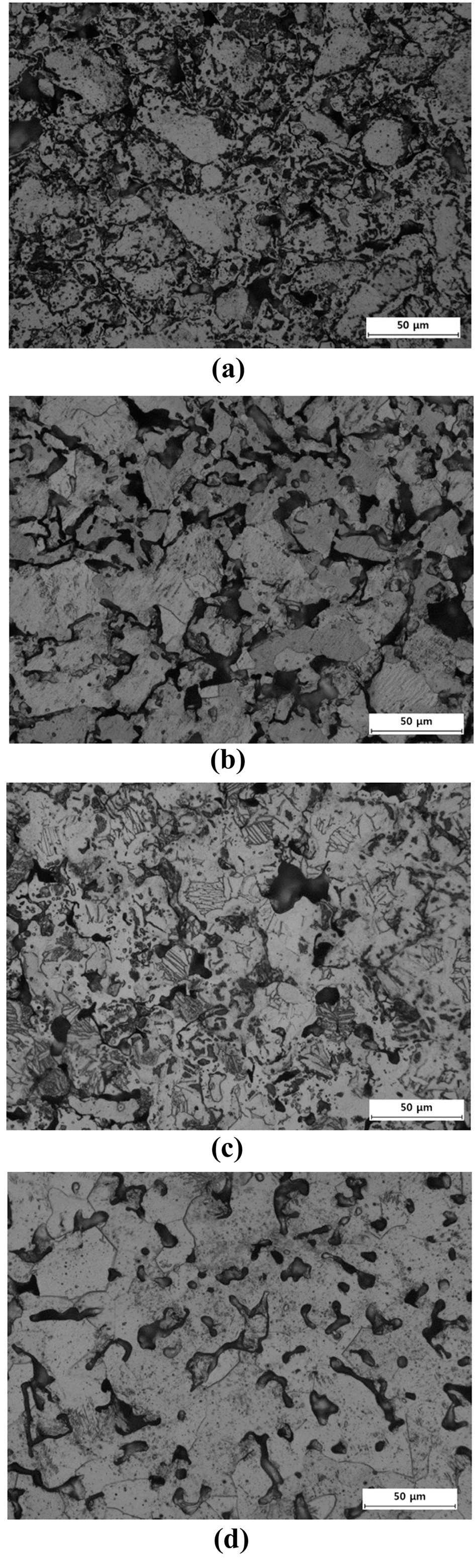
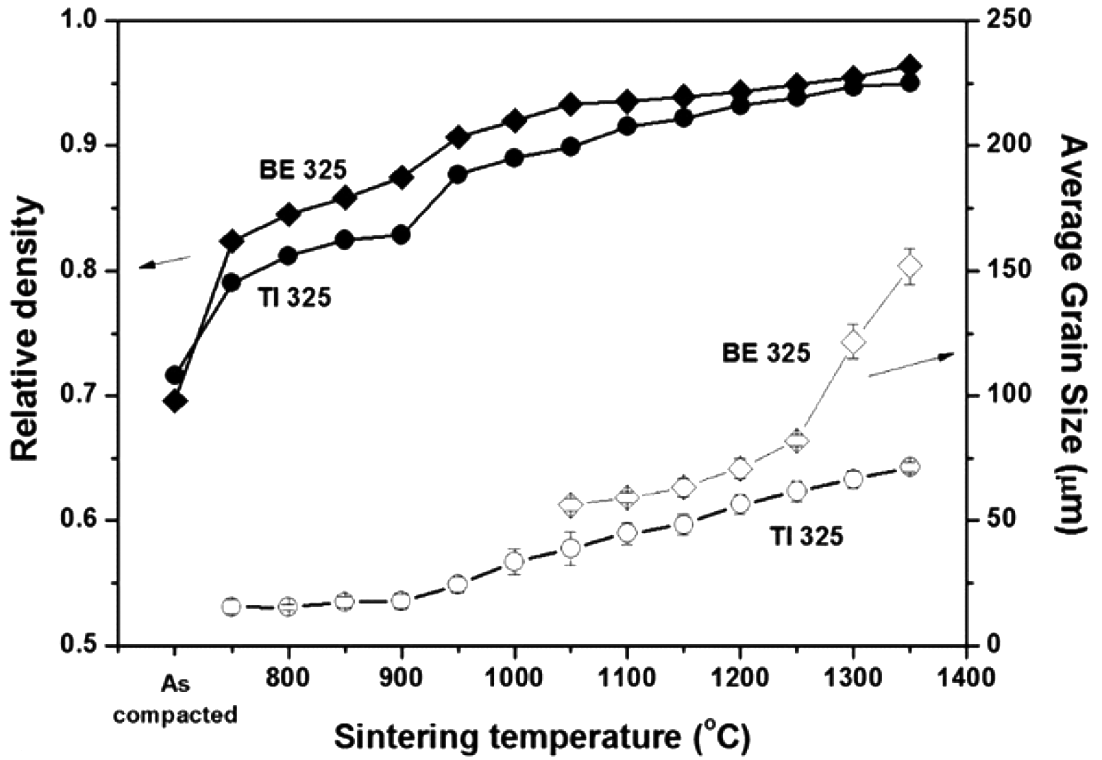
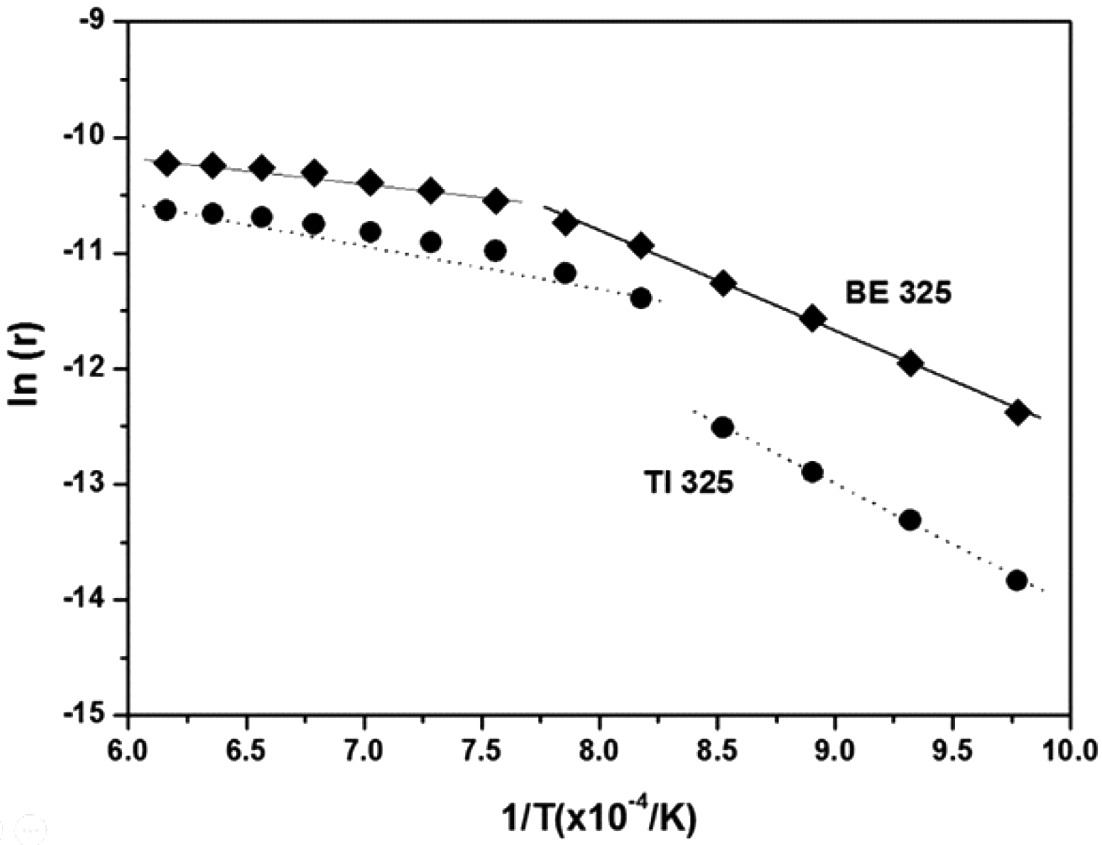


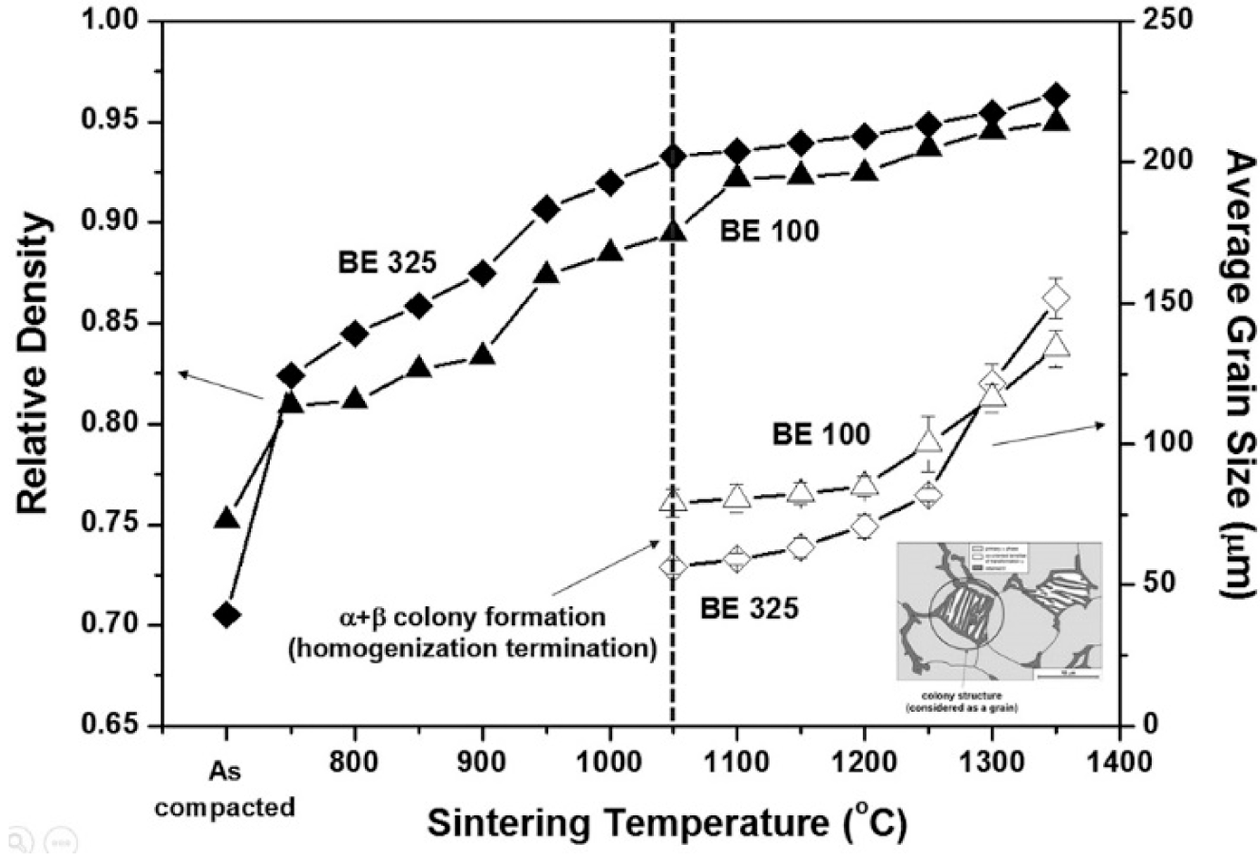
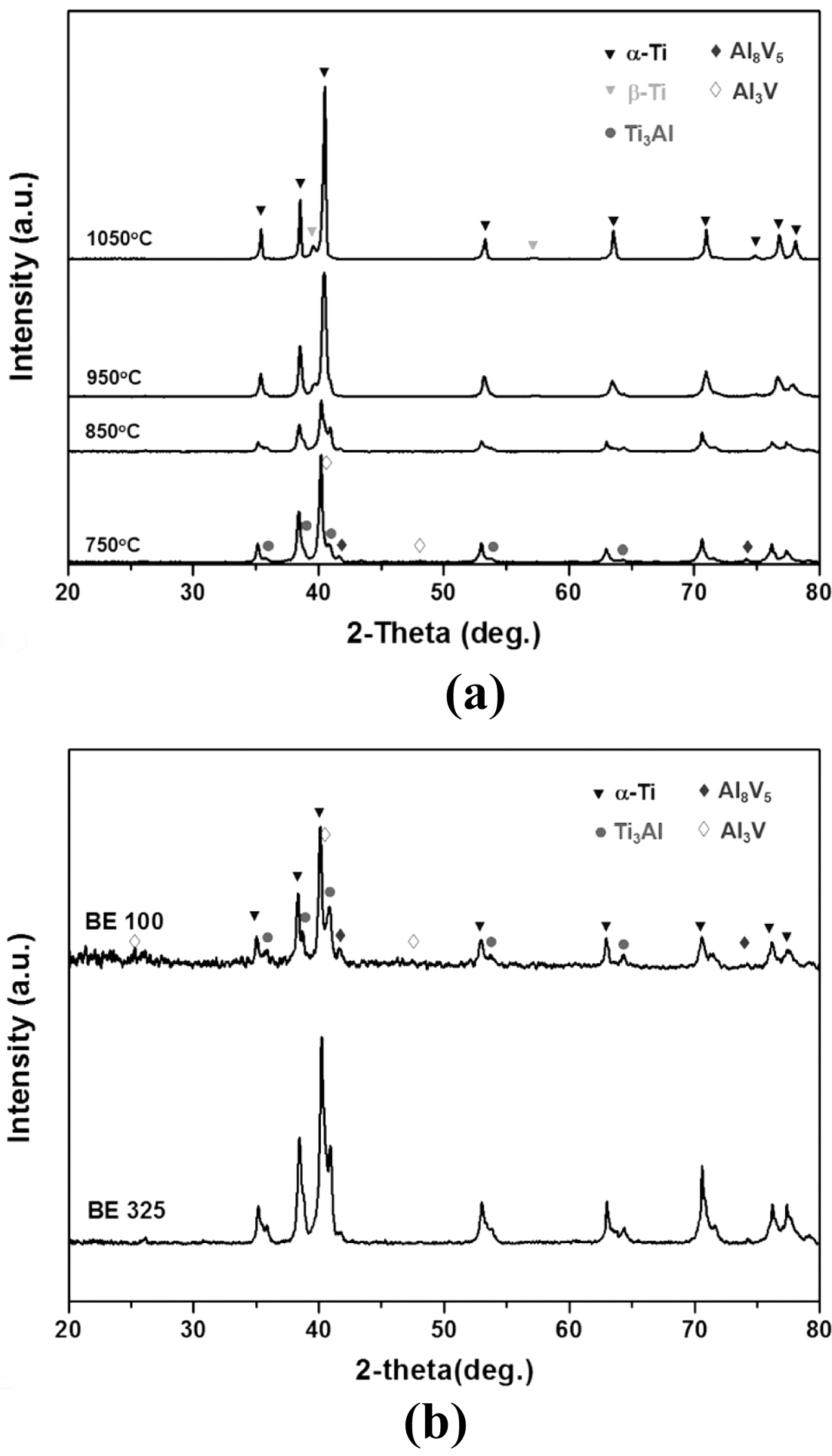
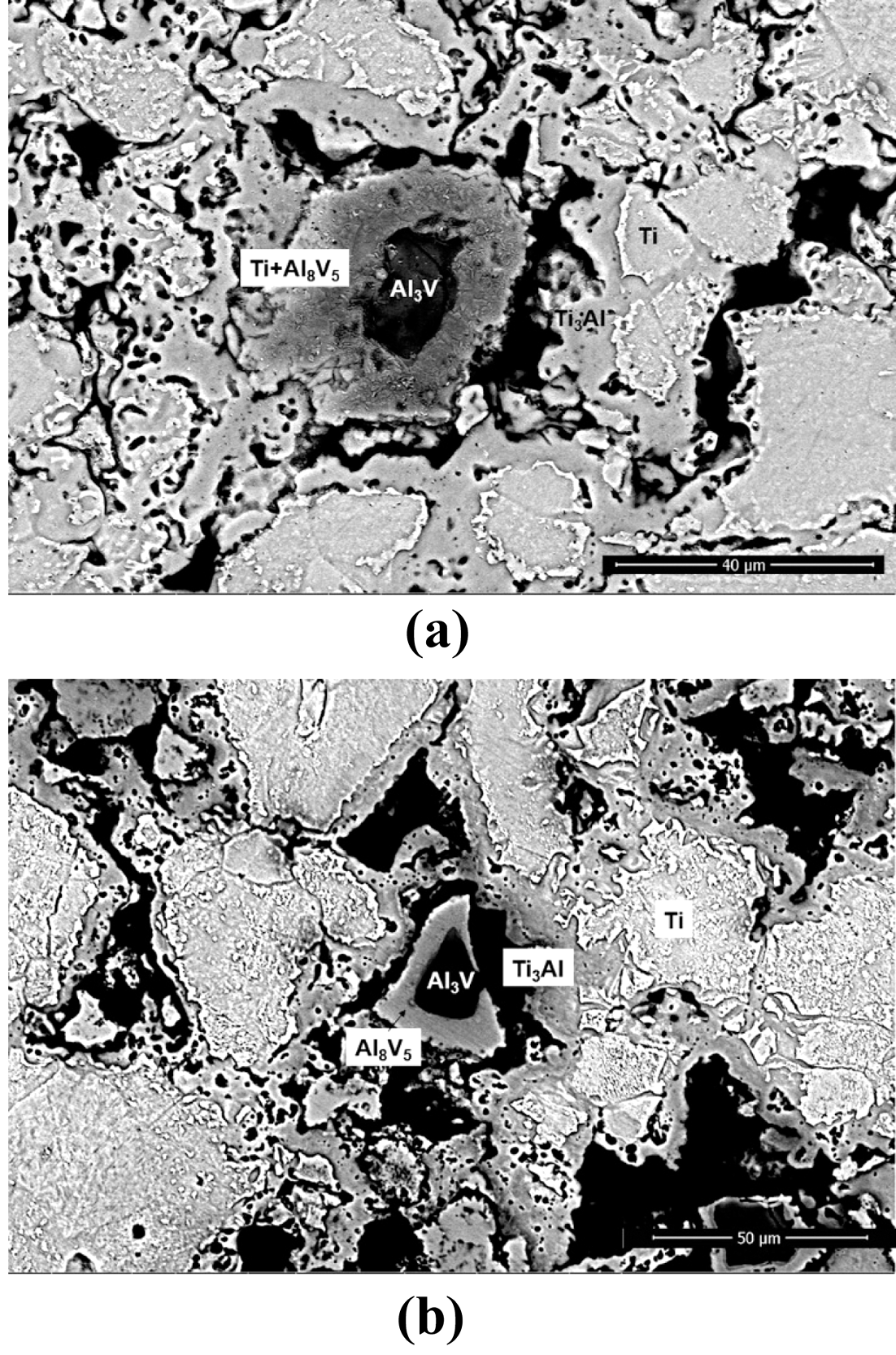


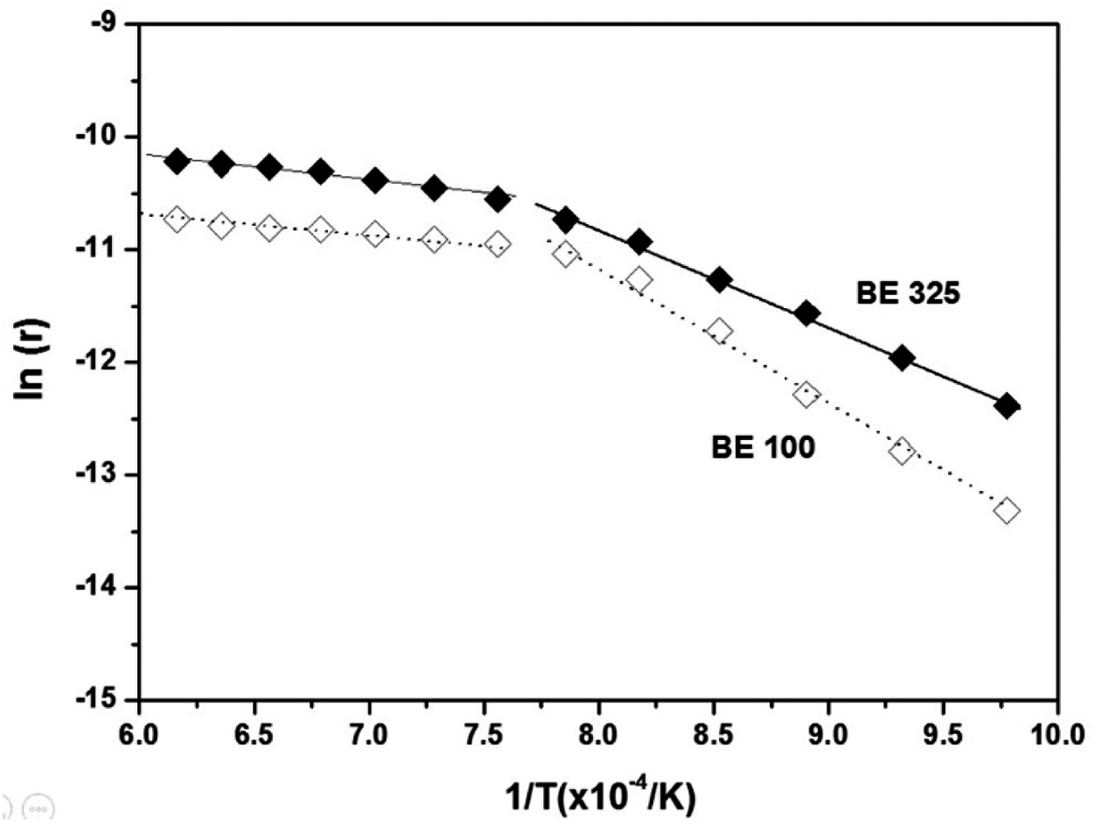
Fig. 1
Scanning electron microscopy (SEM) images showing the morphologies of (a) BE 325, (b) TI 325, and (c) BE 100.
Fig. 2
Shrinkage and shrinkage rates are given versus temperature during heating at 5°C/minutes to 1350°C, and holding at 120 minutes for BE 325 and TI 325.
Fig. 3
Microstructures of sintered samples for BE 325 and TI 325 heated to different temperatures of (a) and (b) 850°C, (c) and (d) 950°C, (e) and (f) 1050°C.
Fig. 4
Variations of the sintered density and average grain size of sintered samples for BE 325 and TI 325 with temperature ranging from 750 to 1350°C.
Fig. 5
Plots of logarithmic shrinkage rate ln (r) versus 1/T to estimate the apparent activation energy for BE 325 and TI 325 compacts.
Fig. 6
Shrinkage and shrinkage rates are given versus temperature during heating at 5°C/minutes to 1350°C, and holding at 120 minutes for BE 325 and BE 100.
Fig. 7
Microstructures of sintered samples for BE 100 heated to different temperatures of (a) 850°C, (b) 950°C, and (c) 1050°C.
Fig. 8
Variations of the sintered density and average grain size of sintered samples for BE 325 and BE 100 with temperature ranging from 750 to 1350°C.
Fig. 9
X-ray diffraction (XRD) patterns of the sintered Ti- 6Al-4V samples for (a) BE 325 heated to 750, 850, 950°C, and 1050°C, and (b) BE 325 and BE 100 heated to 1050°C.
Fig. 10
Backscattered electron images of sintered samples heated to 850°C for (a) BE 325 and (b) BE 100.
Fig. 11
Variation in the amount of constituents with sintering temperature in the vicinity of Al/V mater alloy phase for BE 325 and BE 100.
Fig. 12
Schematic diagrams of phase evolution during heating up to 1050°C for BE 325 and BE 100.
Fig. 13
Plots of logarithmic shrinkage rate ln (r) versus 1/T to estimate the apparent activation energy for BE 325 and BE 100 compacts.
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
Fig. 6
Fig. 7
Fig. 8
Fig. 9
Fig. 10
Fig. 11
Fig. 12
Fig. 13
Solid-state sintering mechanism of blended elemental Ti-6Al-4V powders
| Designation | TI 325 | BE 325 | BE 100 |
|---|---|---|---|
|
|
|||
| Composition (wt%) | 100 Ti | mixed 90 Ti and 6Al/4V | |
| Mean particle size (µm) | 25.93 | 33.60 76.32 | |
| Particle size distribution (µm) | |||
| D10 | 10.6 | 13.12 | 18.17 |
| D25 | 15.84 | 20.11 | 39.18 |
| D50 | 23.01 | 30.93 | 70.53 |
| D75 | 33.88 | 44.47 | 108.2 |
| D90 | 44.84 | 57.24 | 142.4 |
| Morphology | irregular | ||
| Ti | Al | V | Fe | C | N | H | O | |
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| TI325 | Bal. | - | - | 0.05 | 0.02 | 0.02 | 0.009 | 0.09 |
| BE325 | Bal. | 6.30 | 4.10 | 0.06 | 0.01 | 0.02 | 0.012 | 0.16 |
| BE100 | Bal. | 6.45 | 4.03 | 0.05 | 0.01 | 0.01 | 0.010 | 0.13 |
| AMS4998 | Bal. | 5.50-6.75 | 3.50-4.50 | 0.30 | 0.10 | 0.04 | 0.012 | 0.13-0.18 |
| Powder | Temperature Range (°C) | Activation Energy for Sintering (kJ/mol) |
|---|---|---|
|
|
||
| BE 325 | 750-1000 | 135.48 |
| 1050-1350 | 25.08 | |
| TI 325 | 750-900 | 299.35 |
| 950-1350 | 84.45 | |
| BE 100 | 750-1000 | 181.16 |
| 1050-1350 | 17.07 | |
Table 1
Designation and characterization of raw powders
Table 2
Chemical composition of raw powders (unit: wt%)
Table 3
Activation energies for sintering of raw powders with the sintering temperature
Table 1
Table 2
Table 3
TOP
 KPMI
KPMI



 Cite this Article
Cite this Article













