Articles
- Page Path
- HOME > J Korean Powder Metall Inst > Volume 25(2); 2018 > Article
-
ARTICLE
- Synthesis of Boron Nitride Nanotubes via inductively Coupled thermal Plasma process Catalyzed by Solid-state ammonium Chloride
- Mi Se Changa, Young Gyun Nam, Sangsun Yang, Kyung Tae Kim, Ji Hun Yu, Yong-Jin Kim, Jae Won Jeonga,*
-
Journal of Korean Powder Metallurgy Institute 2018;25(2):120-125.
DOI: https://doi.org/10.4150/KPMI.2017.25.2.120
Published online: March 31, 2018
a Metal Powder Department, Korea Institute of Materials Science, 797 Changwondae-ro, Seongsan-gu, Changwon 51508, Korea
- *Corresponding Author: TEL: +82-55-280-3611, FAX: +82-55-55-280-3289, E-mail: jeongjw1204@kims.re.kr
• Received: April 9, 2018 • Revised: April 15, 2018 • Accepted: April 24, 2018
© The Korean Powder Metallurgy Institute. All rights reserved.
- 681 Views
- 7 Download
Abstract
- Boron nitride nanotubes (BNNTs) are receiving great attention because of their unusual material properties, such as high thermal conductivity, mechanical strength, and electrical resistance. However, high-throughput and highefficiency synthesis of BNNTs has been hindered due to the high boiling point of boron (~ 4000°C) and weak interaction between boron and nitrogen. Although, hydrogen-catalyzed plasma synthesis has shown potential for scalable synthesis of BNNTs, the direct use of H2 gas as a precursor material is not strongly recommended, as it is extremely flammable. In the present study, BNNTs have been synthesized using radio-frequency inductively coupled thermal plasma (RF-ITP) catalyzed by solid-state ammonium chloride (NH4Cl), a safe catalyst materials for BNNT synthesis. Similar to BNNTs synthesized from h-BN (hexagonal boron nitride) + H2, successful fabrication of BNNTs synthesized from h-BN+NH4Cl is confirmed by their sheet-like properties, FE-SEM images, and XRD analysis. In addition, improved dispersion properties in aqueous solution are found in BNNTs synthesized from h-BN +NH4Cl.
- Boron nitride nanotube (BNNT), a structural analogue of hexagonal boron nitride (h-BN), is receiving great concerns among material researchers, because of its unusual material properties such as high thermal conductivity [1], high Young’s modulus (up to 1.3 TPa) [2], and high electrical resistance [3], which have never been demonstrated with classical bulky materials, and it is believed to shape the future of mechanical and functional applications.
- BNNTs are structurally similar with carbon nanotubes (CNTs), so many researches have tried to apply synthesis methods which was successful with CNTs. To date, various methods have been introduced to synthesize highly crystalline BNNTs such as chemical vapor deposition (CVD) [4, 5], ball milling [6], arc discharge [7], and laser ablation [8]. However, these methods are limited to synthesizing BNNTs in only gram-level amounts. BNNT synthesis mcethods developed so far are mostly based on seeded-growth mechanism, where boron containing solidphase source are reacted with gas-phase nitrogen which results in successive formation of B-N bonds with hexagonal alternating one-by-one arrangement of boron and nitrogen atoms. In contrast to CNTs, the high-throughput and high-efficiency synthesis of BNNTs was difficult, primarily due to [1] lack of vapor-phase boron precursors, and [2] highly strong bond of nitrogen molecules which hindered fast and efficient reaction between boron and nitrogen atoms. Further study needs to be employed for scalable synthesis of BNNTs along with cost-controlling methods.
- The plasma process system provides a new pathway to the synthesis of scalable and size controllable BNNTs [9]. Extremely high temperature of thermal plasma (exceeding 10000 K) ensures vaporization of boron [10], and strong electric field generated in the plasma torch breaks N-N bond, and makes reactive nitrogen species facilitating B-N reaction. In plasma synthesis of BNNTs, firstly boron nanoparticles are formed via nucleation from highly concentrated boron vapors just after passing high-temperature plasma region. Boron nanoparticles formed during the plasma process act as the seed material for the growth of BNNTs. Because the growth of BNNT starts with the formation of boron seed and ends by the solidification of the boron, the growth of BNNTs is restricted only to temperature range of 2000~4000°C. Boron seed resides in this region very shortly, less than several tens of ms, so there is no plenty of reaction time. Furthermore, most of dissociated nitrogen atoms tend to recombine into N2 just after passing the high-temperature region of plasma, thus the frequency of active reaction between B and N is far below than expected and the growth of BNNTs is disturbed.
- Here, hydrogen plays a big role in impeding the recombination by forming N-H bonds and thus consequently aiding the growth of BNNT as a vital N-source. Kim et al proved the theory through their thermal plasma synthesis of BNNTs with the precursor h-BN and H2 gas [9]. BNNTs made from such precursors showed high yield of nanotubes compared to BNNTs fabricated from h-BN alone. However, direct use of H2 gas as a precursor material is not strongly recommended as it is extremely flammable, having a wide explosive/flammability range (4% - 74% in air), meaning that even a small leak can cause a hazardous fire [11]. Therefore, use of other precursors is recommended for safety reasons, especially when synthesizing materials with high temperature processes.
- Although many methods for synthesizing BNNTs are being developed, further study in BNNT based applications/ devices has not yet been explored extensively due to its difficulty in dispersing in aqueous solution [12]. BNNT solubility region lies at a low hydrogen bonding range, implying its hydrophobicity, thus lacking interaction with solvents with hydroxyl groups [13]. In order to fabricate BNNT composites or to apply to devices, it is crucial to be able to prepare BNNT in uniform and stable dispersions [14].
- Here, we present plasma synthesis of BNNTs in the help of catalytic solid-sate ammonium compounds without using explosive hydrogen. We have chosen NH4Cl as an ammonium compound, because NH4Cl does not leave any solid state byproduct during thermal decomposition, and NH4Cl itself is well dissolvable in pure water. In this work, BNNTs were synthesized via RF-ITP with precursors h-BN, h-BN+H2, and h-BN+NH4Cl. Sheet-like BNNTs were collected from the collector filter only from the two combinations of precursors, h-BN+H2 and h- BN+ NH4Cl, implying successful fabrication of nanotubes. Also meaning that the hydrogen from NH4Cl has substituted the role of H2 gas (impeding the recombination of N2) and contributing to the synthesis of BNNTs. As-synthesized BNNTs were dispersed in distilled water to test for dispersion ability in aqueous solution.
Introduction
- BNNTs were synthesized via TEKNA PL-35LS with precursors h-BN, H2 gas and NH4Cl. Precursors h-BN and NH4Cl were injected from the powder feeder to the plasma torch at feeding rate 4 g/min with 5 slpm of Ar carrier gas. BNNTs were first synthesized from h-BN only. Ar gas was first purged and filled into the system followed by 70 slpm N2 of sheath gas. The second sample of BNNTs was synthesized from precursors h-BN and H2 gas with 5 slpm of Ar carrier gas. The sheath gas was programmed to 65 slpm N2, 15 slpm H2 and 5 slpm of Ar carrier gas. The third sample of BNNTs was also synthesized at the same conditions with a precursor ratio of 1:1 for h-BN and NH4Cl but this time without H2 gas. The two types of powders were manually mixed and were put into the hopper to be injected from the powder feeder. The as-synthesized BNNTs were easily collected from the collector filter as the BNNTs were fabricated into sheet-like form. The aqueous dispersion test of BNNTs was performed by bath sonication for 15 minutes.
- The structural morphology of BNNTs were examined by Field Emission Scanning Electron Microscopy (Tescan, MIRA3 LM) and X-Ray Diffraction (Rigaku, D/ Max-2500VL/PC). Distilled water was used to investigate the dispersion ability of BNNTs in aqueous solution. Vacuum filtration was performed with PTFE filter with sub-20 nm pores.
Experimental
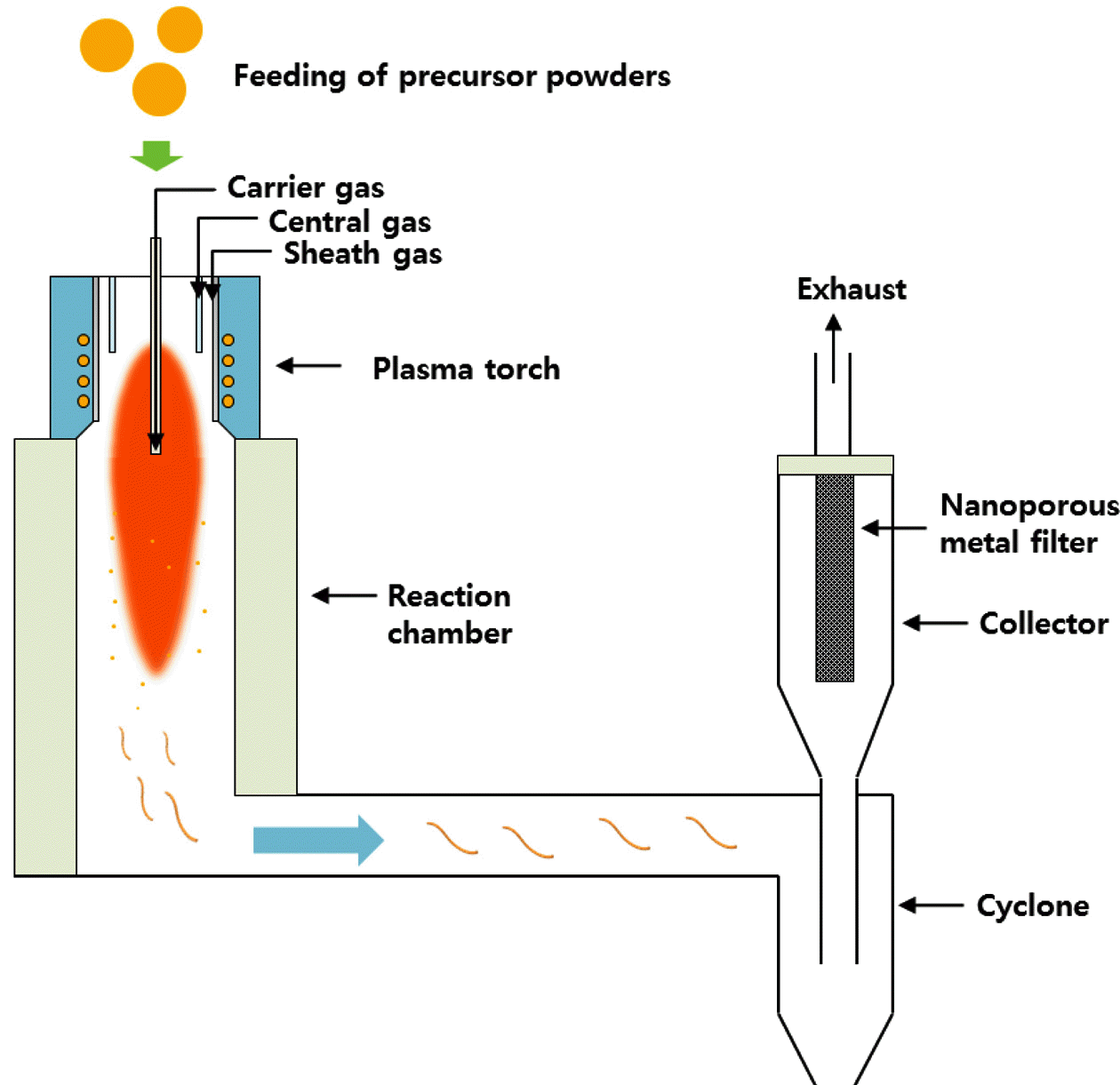
- The RF-ITP system (TEKNA PL-35LS) is arranged into four major parts: the plasma torch, reactor chamber, cyclone chamber, and collector chamber as shown in Figure 1 [15]. Precursor materials are injected into the injection probe and into the thermal plasma created in the plasma torch induced by the induction coil. Figure 2 shows the mechanism scheme of BNNT synthesis via thermal plasma. As shown in figure 2(a), hydrogen from H2 gas plays a role in impeding the recombination of N2 molecule and forming highly reactive N-H molecules, which contribute to the synthesis of BNNTs. Figure 2(b) also shows a similar scheme, however, this time reactive N-H molecules come directly from dissociation of NH4Cl and contribute to the synthesis of BNNTs. Meaning, when NH4Cl is used along with h-BN as a precursor material, H2 gas is no longer needed, solving the safety issues of using H2 gas in extreme temperature synthesis such as thermal plasma. Figure 3 shows photographs of BNNTs synthesized from precursors h-BN+ NH4Cl. Figure 3(a) shows how the sheet-like BNNTs were peeled off from the collector filter. As the sheets are peeled off, the sheets roll up and are easily peeled off from the surface of the filter. Figure 3(b) shows the peeled off and free-standing bundle of sheets of BNNTs. While the BNNTs exhibit grayish color when bundled up, it appears to be whiter when only one layer of BNNTs is peeled off.
- Figure 3 also shows photographs of as-synthesized BNNTs, each synthesized from three different combinations of precursors. Figure 3(c) shows BNNTs synthesized from h-BN only while figure 3(d) and 3(e) shows BNNTs synthesized from h-BN+H2 and h-BN+NH4Cl respectively. A difference can be seen between BNNTs synthesized from only h-BN and when synthesized from H2 or NH4Cl as figure 3(c) does not show any sheet-like properties, and were solely collected in a powder form. However, figure 3d show that BNNTs can fully be grown in our thermal plasma system with the precursor h-BN and H2 gas as reported by Kim et al. [9]. Figure 3(e) also shows that BNNTs can fully be grown from the aid of only NH4Cl. The sheet-like and rolling properties seen in figure 3(b) and 3(c) implies the successful synthesis of nanotubes [16]. The synthesis of BNNTs was possible without H2 gas as NH4Cl played a role in providing hydrogen radicals to form N-H bonds (impeding the recombination of N2 molecule) and thus taking over the role of H2 gas and contributing to the growth of nanotubes. FE-SEM images shown in figure 4(a) and 4(b) confirms the unsuccessful growth of BNNTs from only h-BN as no nanotubes are found in the images. Figure 4(c) and 4(d) show successful growth of BNNTs from h- BN+H2 and same goes with figure 4(e) and 4(f). Figure 4(e) and 4(f) however show a slightly larger portion of nanoparticles distributed between the nanotubes compared to Figure 4(c) and 4(d). While nanotubes in figure 4(c) and 4(d) are entangled with each other, nanotubes in figure 4(e) and 4(f) are more widely distributed, which can improve its dispersion properties when dispersed in aqueous solutions.
- In order to first distinguish the nanoparticles apart from the nanotubes, the as-synthesized BNNTs were characterized by XRD analysis as shown in Figure 4(g). BNNTs synthesized from h-BN and H2 gas only shown in figure 4(g) exhibit h-BN peaks at 2θ = 26, 50° implying hexagonal structures of BNNTs [17]. BNNTs synthesized from h-BN+NH4Cl also exhibit h-BN peaks at 2θ = 26, 50o. In addition, peaks at 2θ = 43, 75o are also seen, indicating the presence of BH12N3O3 [18] (ammonium borate). The presence of above peaks of nanoparticles implies that the nanoparticles distributed between the nanotubes in Figure 4(e)-4(f) are synthesized through the thermal plasma process along with the synthesis of BNNTs. BH12N3O3 [19] possess high solubility in aqueous solution, which may contribute to the dispersion of BNNTs as the distribution of nanoparticles impede the nanotubes from entangling with each other.
- The dispersion ability of the BNNTs samples were tested in distilled water as shown in figure 5. BNNTs synthesized from h-BN+H2 show poor dispersibility in distilled water as shown in figure 5(a). The sheet-like BNNT break into smaller pieces and remain in the solution in a colloidal-like form. BNNTs synthesized from NH4Cl show improved dispersibility in aqueous solution as shown in figure 5(b). Differences can be seen from the two samples as the dispersed BNNTs exhibit opaque properties in figure 5(b) compared to figure 5(a). It is typical to show such opacity in solutions with well-dispersed nanotubes [20], implying that BH12N3O3 nanoparticles played a big role in contributing to the dispersibility properties by impeding the entanglement of BNNTs in the fabrication process. The proposed principle of dispersity enhancement in the help of reaction by product is schematically represented in figure 5(b).
- The dispersed BNNTs can be easily retrieved from the solution by vacuum filtration. Figure 6(a) schematically shows vacuum filtration process employed in this work. BNNT/water solution was poured onto PTFE filter having sub-20 nm pores, and solvents are extracted out via vacuum pumping. Figure 6(b) and 6(c) show optical images of filters poured with solutions dispersed with H2- catalyzed-synthesized and NH4Cl-catalyzed-synthesized BNNTs, respectively. Dark color of collected BNNTs is originated from increased volumetric density of boron nanoparticles due to compaction of BNNTs during filtration process. Figure 6(d, e) and Figure 6(f, g) shows SEM images captured from surfaces of filters decorated with H2-catalyzed-synthesized and NH4Cl-catalyzed-synthesized BNNTs, respectively. Compacted PNNT fibrils can be clearly seen in the images, and it is difficult to find out the difference in areal density of BNNTs between two samples.
Results and Discussion
Fig. 2
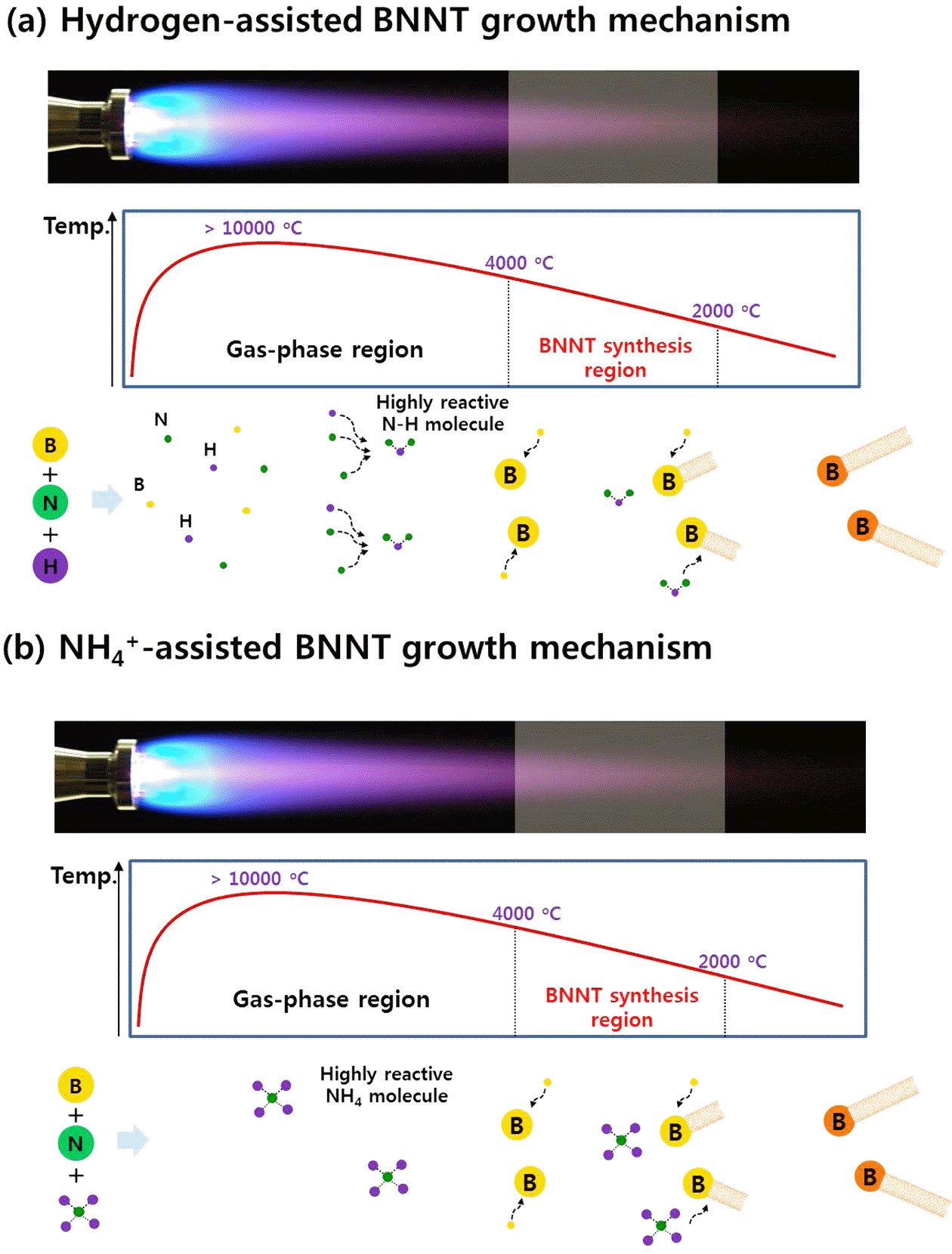
Mechanism scheme of BNNT synthesis with precursors (a) h-BN+H2 (b) h-BN+NH4Cl via thermal plasma.

Fig. 3
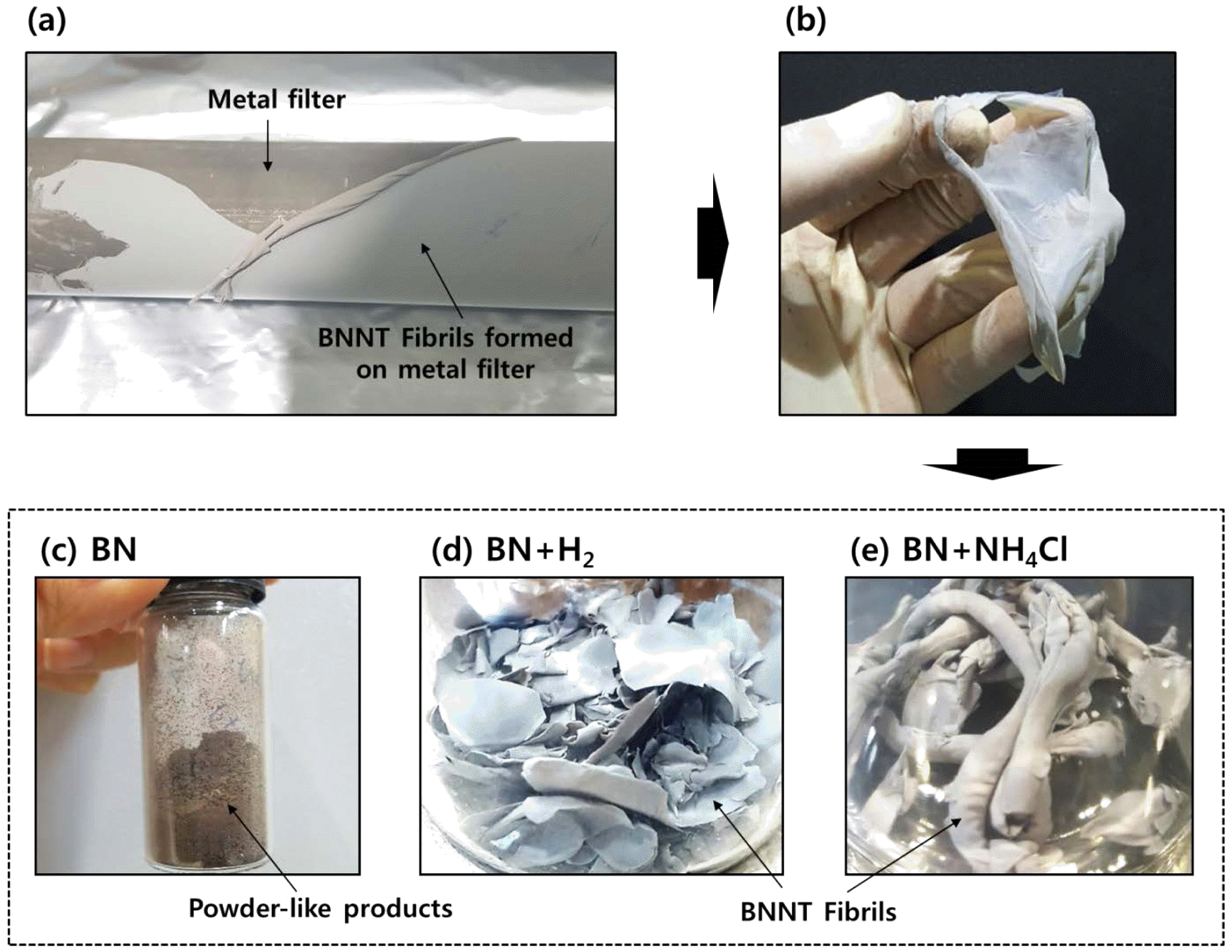
Photographs of as-synthesized BNNTs from precursors h-BN+ NH4Cl via thermal plasma. (a) As-synthesized BNNTs collected on metal filter, and (b) free-standing as-synthesized BNNT sheets peeled off from collector filter. Photographs of BNNTs synthesized from (c) h-BN (d) h-BN+H2 (e) h-BN+NH4Cl.

Fig. 4
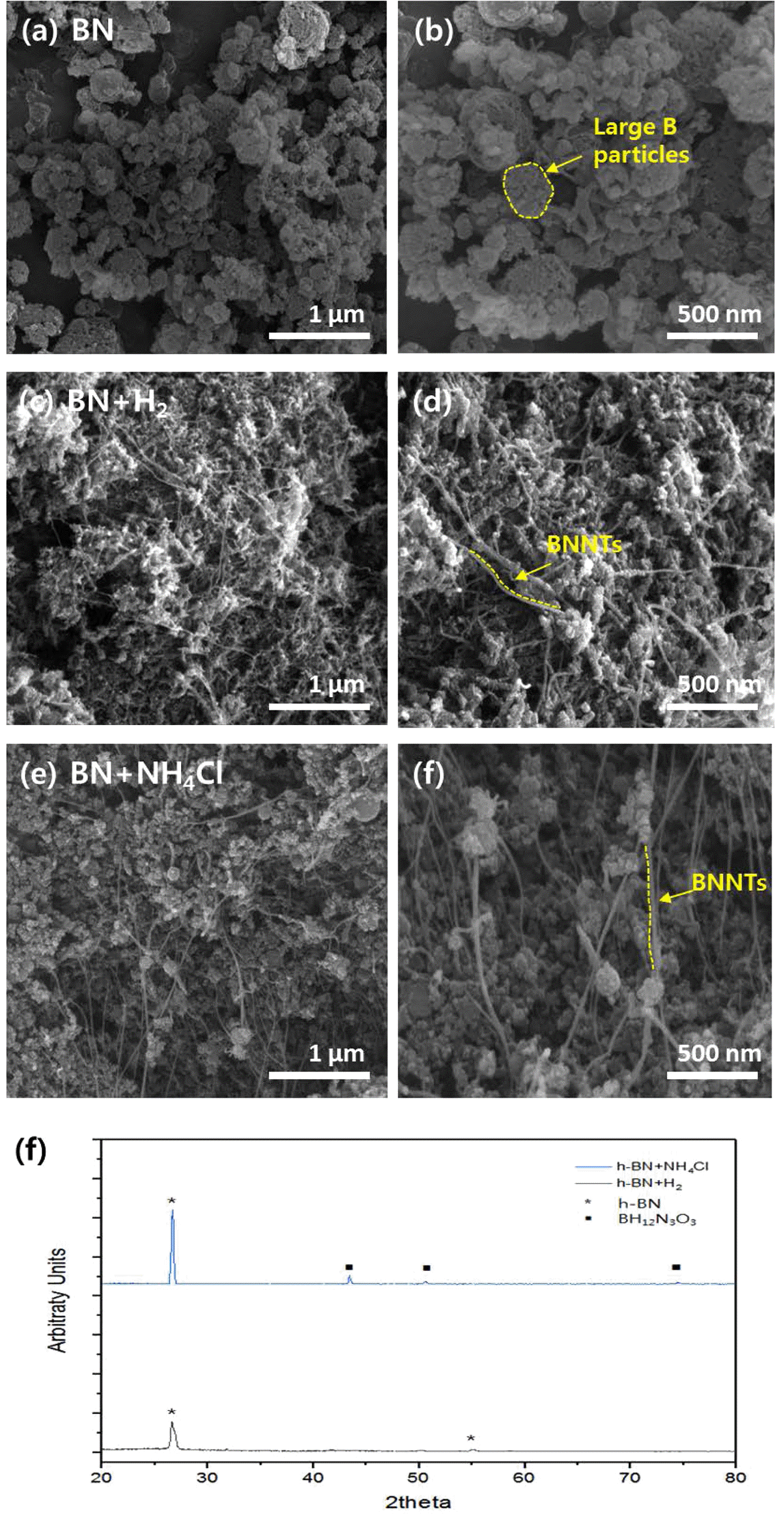
(a-f) FE-SEM images of BNNTs synthesized from (a, b) h-BN (c, d) h-BN+H2 (e, f) h-BN+NH4Cl. (g) XRD analysis result of BNNTs synthesized from h-BN+H2 and h-BN+ NH4Cl.

Fig. 5
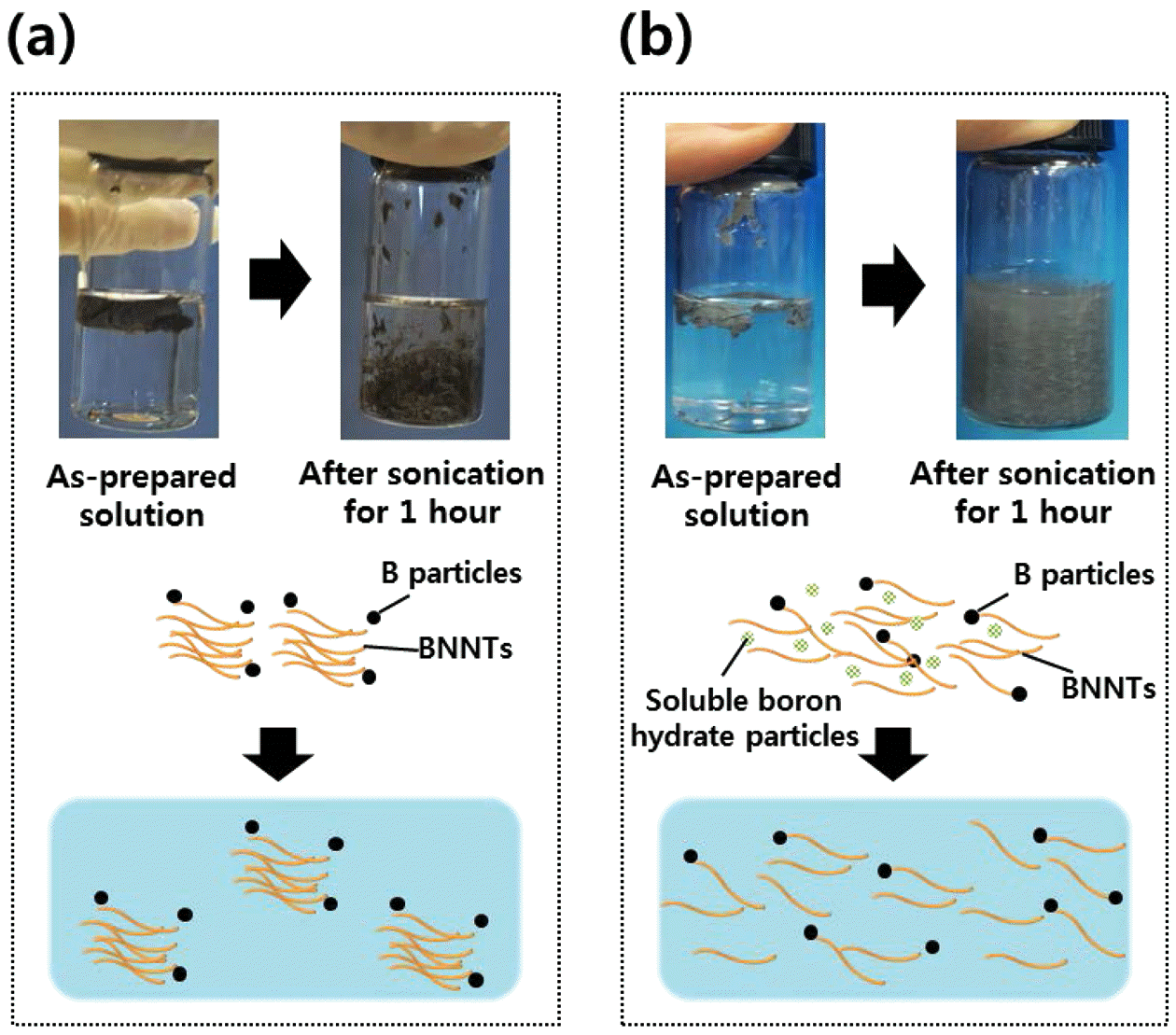
Dispersion test of BNNTs in distilled water synthesized from (a) h-BN+H2 (b) h-BN+NH4Cl, and their schematic representation of dispersion principles.

Fig. 6
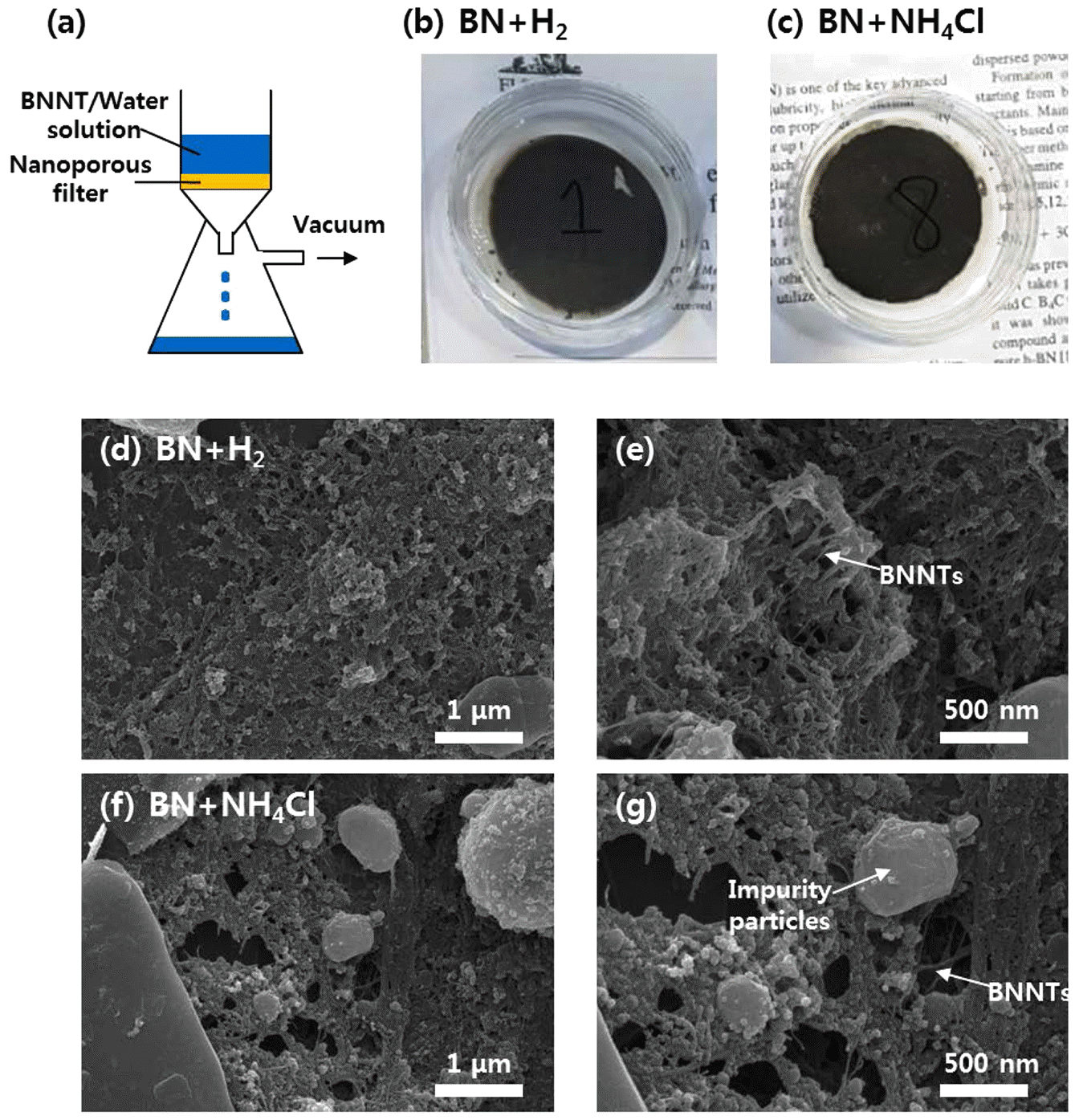
Vacuum filtration process of BNNT solution. (a) A schematic of vacuum filtration process. (b, c) Optical images of filters poured with solutions dispersed with H2-catalyzedsynthesized (b) and NH4Cl-catalyzed-synthesized BNNTs (c). (d-g) SEM images captured from surfaces of filters decorated with H2-catalyzed-synthesized (d, e) and NH4Clcatalyzed- synthesized BNNTs (f, g).

- BNNTs were synthesized from three different combinations of precursors via thermal plasma. Because of the safety reasons of using H2 gas in high temperature synthesis, the use of different precursor other than H2 gas was investigated. h-BN was used as the base precursor with H2 and NH4Cl as the independent variable. BNNTs synthesized from NH4Cl (without H2 gas) showed successful fabrication of nanotubes with sheet-like and rolling properties. In addition, BNNTs synthesized from NH4Cl showed improved dispersibility in aqueous solution compared to nanotubes synthesized from h-BN+H2 gas. The BNNTs successfully retrieved from the solution by vacuum filtration.
Conclusion
-
Acknowledgements
- This study was supported financially by Fundamental Research Program of the Korea Institute of Materials Science (KIMS).
Acknowledgements
- 1. C.W. Chang,, A.M. Fennimore,, A. Afanasiev,, D. Okawa,, T. Ikuno,, H. Garcia,, D. Li,, A. Majumdar, and A. Zettl:: Phys. Rev. Lett.., 97 (2006) 085901.
- 2. N.G. Chopra, and A. Zettl:: Solid State Commun.., 105 (1998) 297.
- 3. C.H. Lee,, M. Xie,, V. Kayastha,, J. Wang, and Y.K. Yap:: Chem. Mater.., 22 (2010) 1782.Article
- 4. O.R. Lourie,, C.R. Jones,, B.M. Bartlett,, P.C. Gibbons,, R.S. Ruoff, and W.E. Buhro:: Chem. Mater.., 12 (2000) 1808.Article
- 5. M.J. Kim,, S. Chatterjee,, S.M. Kim,, E.A. Stach,, M.G. Bradley,, M.J. Pender,, L.G. Sneddon, and B. Maruyama:: Nano Lett.., 8 (2008) 3298.Article
- 6. L. Lu Hua,, C. Ying, and M.G. Alexey:: Nanotechnology., 21 (2010) 105601.Article
- 7. A. Loiseau,, F. Willaime,, N. Demoncy,, G. Hug, and H. Pascard:: Phys. Rev. Lett.., 76 (1996) 4737.Article
- 8. D.P. Yu,, X.S. Sun,, C.S. Lee,, I. Bello,, S.T. Lee,, H.D. Gu,, K.M. Leung,, G.W. Zhou,, Z.F. Dong, and Z. Zhang:: Appl. Phys. Lett.., 72 (1998) 1966.
- 9. K.S. Kim,, C.T. Kingston,, A. Hrdina,, M.B. Jakubinek,, J. Guan,, M. Plunkett, and B. Simard:: ACS Nano., 8 (2014) 6211.Article
- 10. K. Keun Su,, K. Myung Jong,, P. Cheol,, C.F. Catharine,, C. Sang-Hyon, and T.K. Christopher:: Semicond. Sci. Technol.., 32 (2017) 013003.ArticlePDF
- 11. Fact Sheet:, Hydrogen Safety [Internet]. Office of Environment, Health & Safety. 2013..
- 12. C. Lee,, S. Bhandari,, B. Tiwari,, N. Yapici,, D. Zhang, and Y. Yap:: Molecules., 21 (2016) 922.Article
- 13. In : . Boron nitride nanotube: synthesis and applications. SPIE Smart Structures and Materials + Nondestructive Evaluation and Health Monitoring; 2014: SPIE..
- 14. G. Ciofani,, G.G. Genchi,, I. Liakos,, A. Athanassiou,, D. Dinucci, and F. Chiellini V.Mattoli:: Mattoli : J. Colloid Interface Sci., 374 (2012) 308.
- 15. B. Todorović-Marković,, Z. Marković,, I. Mohai,, Z. Nikolić,, Z. Farkas,, J. Szépvölgyi,, E. Kovats,, P. Scheier, and S. Feil:: J. Phys. D.., 39 (2006) 320.
- 16. C. Zhi,, Y. Bando,, C. Tang, and D. Golberg:: Mater. Sci. Eng. R Rep.., 70 (2010) 92.
- 17. T.H. Ferreira,, D.C.F. Soares,, L.M.C. Moreira,, P.R.O. da Silva,, R.G. dos Santos, and E.M.B. de Sousa:: Mater. Sci. Eng. C., 33 (2013) 4616.
- 18. J. Hannauer,, U.B. Demirci,, C. Geantet,, J.M. Herrmann, and P. Miele:: Phys. Chem. Chem. Phys.., 13 (2011) 3809.
- 19. Boron Specialties. Available from:. http://www.boron.com/ammonia_borane.html
- 20. J. Yu,, Y. Chen, and B.M. Cheng:: Solid State Commun.., 149 (2009) 763.
Figure & Data
References
Citations
Citations to this article as recorded by 

Synthesis of Boron Nitride Nanotubes via inductively Coupled thermal Plasma process Catalyzed by Solid-state ammonium Chloride





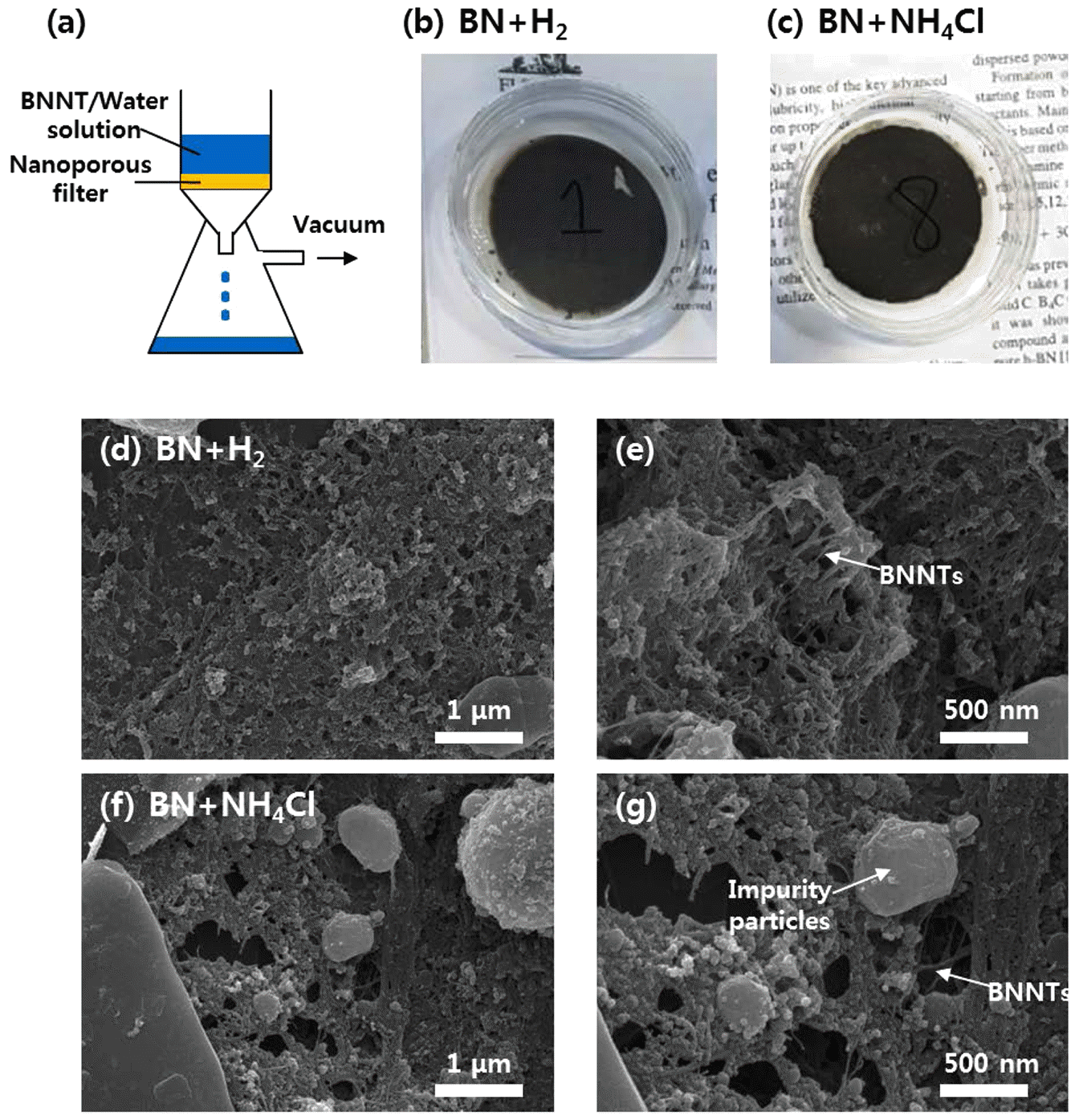
Fig. 1
Schematic illustration of RF-ITP system employed in this work.
Fig. 2
Mechanism scheme of BNNT synthesis with precursors (a) h-BN+H2 (b) h-BN+NH4Cl via thermal plasma.
Fig. 3
Photographs of as-synthesized BNNTs from precursors h-BN+ NH4Cl via thermal plasma. (a) As-synthesized BNNTs collected on metal filter, and (b) free-standing as-synthesized BNNT sheets peeled off from collector filter. Photographs of BNNTs synthesized from (c) h-BN (d) h-BN+H2 (e) h-BN+NH4Cl.
Fig. 4
(a-f) FE-SEM images of BNNTs synthesized from (a, b) h-BN (c, d) h-BN+H2 (e, f) h-BN+NH4Cl. (g) XRD analysis result of BNNTs synthesized from h-BN+H2 and h-BN+ NH4Cl.
Fig. 5
Dispersion test of BNNTs in distilled water synthesized from (a) h-BN+H2 (b) h-BN+NH4Cl, and their schematic representation of dispersion principles.
Fig. 6
Vacuum filtration process of BNNT solution. (a) A schematic of vacuum filtration process. (b, c) Optical images of filters poured with solutions dispersed with H2-catalyzedsynthesized (b) and NH4Cl-catalyzed-synthesized BNNTs (c). (d-g) SEM images captured from surfaces of filters decorated with H2-catalyzed-synthesized (d, e) and NH4Clcatalyzed- synthesized BNNTs (f, g).
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
Fig. 6
Synthesis of Boron Nitride Nanotubes via inductively Coupled thermal Plasma process Catalyzed by Solid-state ammonium Chloride
TOP
 KPMI
KPMI

 Cite this Article
Cite this Article






