Articles
- Page Path
- HOME > J Korean Powder Metall Inst > Volume 25(6); 2018 > Article
-
ARTICLE
- Nanodiamonds Conjugated with Nonsteroidal Anti-inflammatory Drugs for Transdermal Delivery
- Changkyu Rheea,*, Alexey P. Puzyrb, Andrey E. Burovb,c, Olga G. Burovad, Whungwhoe Kima, Vladimir S. Bondarb
-
Journal of Korean Powder Metallurgy Institute 2018;25(6):459-465.
DOI: https://doi.org/10.4150/KPMI.2018.25.6.459
Published online: November 30, 2018
a Nuclear Materials Research Division, Korea Atomic Energy Research Institute (KAERI), Daejeon 305-353, Republic of Korea
b Institute of Biophysics, Siberian Branch of Russian Academy of Science, Krasnoyarsk Science Center SB RAS, 660036 Krasnoyarsk, Russia
c Institute of Computational Technologies, Siberian Branch of Russian Academy of Science, 660049 Krasnoyarsk, Russia
d School of Architecture and Design, Siberian Federal University, 660041 Krasnoyarsk, Russia
- *Corresponding Author: Changkyu Rhee, TEL: +82-42-868-8551, FAX: +82-42-868-8275, E-mail: ckrhee@kaeri.re.kr
© The Korean Powder Metallurgy Institute. All rights reserved.
- 805 Views
- 1 Download
- 1 Crossref
Abstract
- Most commercially available detonation nanodiamonds (DNDs) require further processing to qualify for use in biomedical applications, as they often contain many impurities and exhibit poor dispersibility in aqueous media. In this work, DNDs are modified to improve purity and impart a high colloidal stability to the particles. The dispersive and adsorption properties of modified DNDs are evaluated in terms of the suitability of DNDs as carriers for non-steroidal anti-inflammatory drugs (NSAIDs) in transdermal delivery. The study of adsorption on strongly positively and strongly negatively charged DNDs showed their high loading capacity for NSAIDs, and a pronounced relationship between the drugs and the particles’ charges. Experiments on long-term desorption carried out with DND/NSAID complexes indicate that the nanoparticles exert a sustained effect on the drug release process.
- Detonation nanodiamonds (DND) are receiving much attention due to its remarkable mechanical, electrical and thermal properties, and large specific area. Nanodiamonds find wide applications in composite coatings for metals, diagnostic probes, micro-electro-mechanical devices, additives to polymers and lubricants etc [1-7]. The modifiable surface, high adsorption capacity, non-toxicity and small size of nanodiamond particles suggest their great potential in the biomedical field, particularly in drug delivery. The capacity of DND in drug delivery has been demonstrated many times for a variety of compounds employing different loading mechanisms [1-6]. The most important advantages of DND as a drug delivery vehicle stem from a strong physical adsorption controlled by the functional groups on well-developed surface area that is well suited for conjugating various biochemical substances. A high loading capacity means a high concentration of payload to be delivered using lower concentrations of DND. However, to fully use the potential of DND in drug delivery, attention must be paid to its purity, surface chemistry, colloidal stability and other parameters that may influence drug adsorption and desorption.
- To be used in biomedical applications, most of commercial DND require further processing as they contain a high amount of impurities, are highly polydispersed and often exhibit micron-sized clusters unbreakable by the ultrasonic treatment [8-9]. Upon drying, DND particles aggregate and lose colloidal stability, which makes impossible their subsequent re-suspension or fractionating. There are several ways to overcome the aggregation and improve sedimentation stability of DND including the use of surfactants, removing impurities with ion exchange resins, oxidation at elevated temperatures, and ultrasonic irradiation [1, 8, 10-12]. However, most of them involve high power consumption and the use of costly equipment and reagents.
- In this study we propose a simple and cost effective method for modifying commercially available DND with aims to provide properties suitable for drug delivery, particularly for the use of DND as a carrier for non-steroidal anti-inflammatory drugs. The dispersive and adsorption properties of modified DND were evaluated on purpose to be exploited in controlled drug release for prolonged times.
- Non-steroidal anti-inflammatory drugs (NSAID) are a group of compounds known to prevent formation of prostaglandins and thromboxanes from arachidonic acid through the inhibition of the enzyme cyclo-oxygenase [13]. The drugs of this group are widely used for acute and chronic diseases accompanied by pain and inflammation, with their oral, intramuscular or intravenous administration. However, there are a number of undesirable side effects associated, primarily with the digestive tract and kidneys. An alternative way to deliver NSAID is to use them in medical patches intended for external use with controlled and sustained release of the drug [14]. Diclofenac and Ketorolac, which are widely used in surgery, traumatology, neurology, gynecology, urology, oncology and other medical fields, were chosen for the purposes of this research. Both drugs are hydrophobic, but they are soluble in water in salt form.
Introduction
- Detonation nanodiamond materials from three suppliers were used in this study: powder grade 0-150 produced by Real-Dzerzhinsk Ltd., Russia (hereafter named RND); powder obtained upon drying 5% nanodiamond aqueous dispersion produced by Carbodeon Ltd Oy, Finland (CND); powder produced by SKN, Snezhinsk, Russia (SND). The reason behind the choice of DND was to study the surface charge effect on the binding capacity of DND for the therapeutic agents. CND and SND have a positive zeta potential, while RND shows a negative charge in aqueous solvents.
- Ketorolac Tromethamine and Diclofenac sodium with molecular weight of 376.4 and 318.1, respectively, were obtained from Sigma-Aldrich Co. LLC and used without further purification.
- Modification of the initial DND was performed to obtain material having a high colloidal stability and purity required for biomedical application. Sodium bicarbonate (NaHCO3) was found to show a good effectiveness as a modifier for the chosen materials. The process of modification and simultaneous fractionating started with preparation of an initial suspension, which includes deionized water DI (18 MΩ cm, Milli-Q system, USA), a DND powder in concentration of 5 wt% and the modifying reagent. Ultrasonic pretreatment of the initial DND suspensions was not performed as sonication may result in wear debris from the sonotrode tip, which are difficult to remove. The suspension was held at room temperature for 5 hours, during which DND particles precipitated and reaction products between the modifier and surface substances of DND released into the supernatant. The precipitate was treated with water, intensively agitated with a stirrer and then centrifuged at 5000 g for 5 min at 10oC using the Eppendorf Centrifuge 5415 R (Eppendorf AG, Germany). The residue was added with water and stirred to get a uniform solution. The suspension was centrifuged at 16000 g for 20 min at 10oC and the resulting supernatant was collected. The cycle was repeated until the excess of modifier was removed (it manifested as a change of the supernatant color due to floating DND particles). Then the centrifugal fractionation of samples was conducted at 10oC with the following regimes: at 16000 g for 10 min; at 10000 g for 10 min; at 5500 g for 20 min; at 1000 g for 20 min. The final products of the modification procedure are suspensions of DND particles, whose size distribution depends on acceleration used during centrifugation. These suspensions were dried, the powders were weighed and the weight percents of each fraction were determined. Then all fractions of a certain DND were combined thus obtaining a sample to be used in experiments.
- It is well known that DND enriched with carboxyl groups exhibits high affinity to proteins and polypeptides [4]. However, for a high effectiveness the DND surface should be as uniform as possible. RND powder was used to produce the carboxylated nanodiamond (RND-COOH). The functionalization was achieved chemically employing the procedure described in [15] with a slight modification. An aqueous suspension of RND (3.0 g) was added to 100 ml of a preheated (60°C) mixture of H2SO4 : HNO3 (3:1) and stirred for 48 h. Then, to remove the excessive acid, 50 ml of 0.1 M aqueous NaOH were added and the mixture was stirred at 80°C for 3 hours following the addition of 0.1 M aqueous HCl for 2 hours. Finally, the resulting mixture was extensively washed several times by centrifugation with deionized water. The obtained sediment of RND-COOH was dried and dispersed in DI water for the further modification by the procedure described above. It should be noted that aqueous suspensions obtained from a dry powder of RND-COOH do not exhibit colloidal stability.
- Unimodal particle size distributions of DND (pristine and NSAID-loaded) were determined with the dynamic light scattering technique using a Zetasizer Nano ZS (Malvern Instruments Ltd, UK). The same device was used for the zeta (electrokinetic) potential measurements. The data given are averaged over 3 measurements, 30 runs each performed on ~0.1 wt.% ND aqueous suspensions at 25°C. The elemental composition of DND was examined using a scanning electron microscope (Hitachi TM-1000, Japan).
- To obtain DND-NSAID complex, 100 μl of RND, RND-COOH, SND or CND suspension (5 mg of powder) was added to 1000 μl aqueous solutions of known concentrations of NSAID into Eppindorf vials. The vials were shaken at ambient temperature for 10 min to ensure equilibrium adsorption. Then 2 μl of CaCl2 (1M) or 0.25 M NaCl solution was added to the mixture and kept for 5 min under continual stirring. To precipitate the nanodiamond particles before the absorbance measurements were made, the resulting mixture was centrifuged at 16000 g for 10 min at 10°C. The peak absorbance of the supernatants with nonadsorbed NSAID was measured at 276 and 323 nm (correspondingly, for Diclofenac and Ketorolac) by using a UV-1800 (Shimadzu, Japan) spectrophotometer. The supernatants were diluted 1:400 before being analyzed. The drug concentration was determined by the Beer’s law using a linear calibration. Calibration curves were obtained by a series of separate measurements on the solutions without DND with known concentrations of the drug. The drug binding (adsorption) capacity of DND was calculated using the following equation:
- where Wd - the weight of the drug adsorbed, WDND - the weight of DND nanoparticles.
- The diffusion profiles of RND–COOH/Diclofenac and CND/Ketorolac complex were evaluated in vitro at 26°C employing a home-made cell assembled with a semi-permeable membrane between the donor and receptor chambers. The choice in favor of RND–COOH rather than RND was determined by the higher loading capacity of the former.
- A dialysis tubing 36/32 VISKING (Serva, Germany) with the overage pore radius of 24 Å and exclusion limit of 8-15 kDa was used as the membrane. Before the experiment, the dialysis bag was activated with 10 mM EDTA-Na salt (pH 7.0) for 1 h at 50°C and then washed several times in DI water. Preliminary spectrophotometric testing showed that no DND particles could pass the barrier. The donor chamber was a plastic ring (27 mm in diameter) covered by the dialysis bag. The sample under study (2 ml) were introduced into the donor chamber, one end of which was fastened to a float immersed in a glass beaker filled with 30 ml of DI water (receptor solution). Continuous stirring was maintained throughout the testing using a magnetic bar. The receptor solution was periodically withdrawn (each hour during the first 24 hours, then with 12 hour interval) and replaced with equal volume of DI water. The drug concentration in the receptor solution was determined at the absorption maxima using the calibration curves. As a control, a release pattern of free drugs dissolved in 2 ml of DI water was monitored under the same conditions. The drug payload was virtually the same in the test and control samples. For the former, it was calculated based on the drug loading capacity of DND determined by absorption tests.
Experimental
- The stability of DND suspensions prepared from the as-received powders was low. After storage for ca. 1 hour they separated into observable layers containing aggregates of different sizes formed under the influence of gravity. Metallic impurities, which facilitate coagulation of individual diamond particles, may be one of the reasons for poor dispersion [16]. In contrast, the suspensions of modified DND exhibited a good colloidal stability with no precipitation observed for at least 6 months. They retained their colloidal stability after several cycles of drying followed by the addition of water to the resulting powder. All tested materials exhibit a feature that is intrinsic for DND: the color of a powder depends on the average size of particles. It changes from a deep black for the smallest fraction through grey for the middle sized fraction to milk white or beige for the biggest one [17]. The modification significantly reduces amounts of inorganic impurities contained in the initial DND as it was showed by the elemental analysis. For example, the total amount of metals (Al, Si, P, Ca, Fe, Ni, Cu and Zn) in RND samples was reduced by more than two times (from 1.15% to 0.47%). We attribute such improvement to the use of modifier that binds ions of bivalent metals.
- Colloidal stability can be measured quantitatively through the electrokinetic potential (zeta potential). All modified DND have a measured z-potential above 30 mV or below −30 mV (Table 1) and are considered stable because these particles presumably maintain their repulsive forces while dispersed [17]. It is important to note that the performed modification does not alter the sign of charge of DND particles in suspension, but increase the change degree (in absolute value).
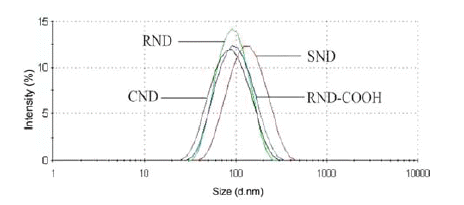
- The fraction analysis revealed that studied DND are polydispersed systems containing DND aggregates of different sizes (Fig. 1). All but SND samples displayed comparable average size and distribution of nanoparticles. It implies that the size effect on the drug loading capacity of these materials was not significant.
- Processing of the soot or further oxidation of DND (like carboxylation) results in oxidation with predominant carboxylic acid groups on the DND surface. Dissociation of these groups in water leads to a negative charge on surfaces of RND and RND-COOH. The positive charges of CND and SND can be attributed to protonation of amino groups on the DND surfaces.
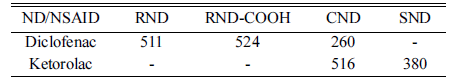
- The adsorption study showed a significant difference of DND in the adsorption capacity to the NSAID as it is demonstrated in Table 2.
- The data show that negatively charged DND (RND and RND-COOH) practically do not adsorb Ketorolac, which has a negative charge in water, due to electrostatic repulsion. In contrast, these DND demonstrated a high adsorption to positively charged Diclofenac with a comparable capacity. RND-COOH showed a slightly higher value, presumably due to differences in surface chemistry, since RND-COOH has a higher density of negative surface groups.
- The values of the zeta potential (Table 1) for RND, RND-COOH and their conjugates with Diclofenac indicate the presence of the drug on the DND surface. Due to the formation of complexes, the zeta potential of both RND/Diclofenac and RND-COOH/Diclofenac conjugates was significantly lower in absolute magnitude than that of the pristine DND. The same way, the conjugation of Ketorolac to the CND surface decreased the positive charge of DND by about 50%, which resulted in a low stability of the complexes in suspension. Adsorption of Diclofenac also manifests in an increase in the size of RND particles by 50% (Table 1). Surprisingly, the adsorption of the drug on RND-COOH resulted in a reduction (from 83 to 66 nm) of the average particle size. Such a phenomenon we observed in our earlier studies when bovine serum albumin was adsorbed on RNDCOOH.
- An attempt has been made to improve the adsorption capacity of RND-COOH for Ketorolac by changing the pH of the solution. However, the transition to alkaline medium did not lead to a change in the adsorption capacity, while even a slight decrease in pH led to the crystallization of the drug, which made it impossible to carry out the experiment.
- Positively charged CND and SND showed a high adsorption of Ketorolac, although their binding capacity was different (Table 2). At the same time, SND did not adsorb Diclofenac molecules. It is widely recognized that electrostatic forces play an important role in the adsorption process on DND. However, the electrostatic attraction/ repulsion model cannot explain the observed interactions between CND and Diclofenac [18]. The binding capacity of CND for Diclofenac was almost twofold less than that of RND or RND-COOH, however, it was still quite high.
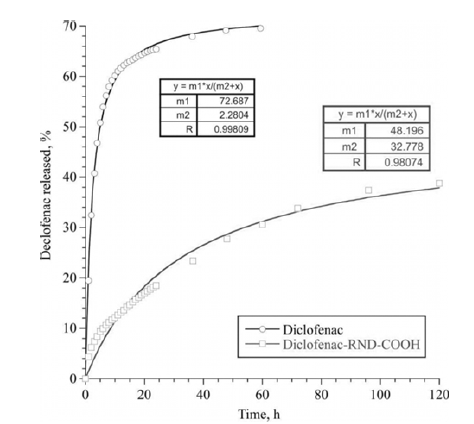
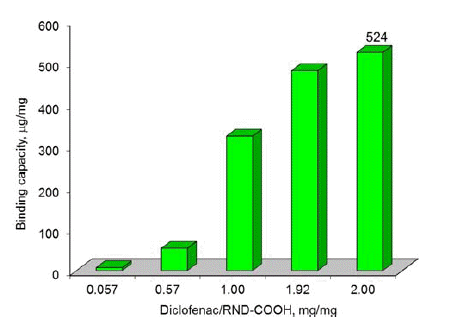
- Figures 2-3 depict the results of determining the binding capacity for RND and RND-COOH, depending on the mass ratio of Diclofenac and nanoparticles. As follows from the data presented, the binding capacity of DND increases with increase of the mass ratio, reaching the maximum values of 511 and 524 μg/mg, respectively. Given that the size of the DND particles is much larger than the size of the drug molecule, it is obvious that during adsorption the latter form several layers on the surface of DND.
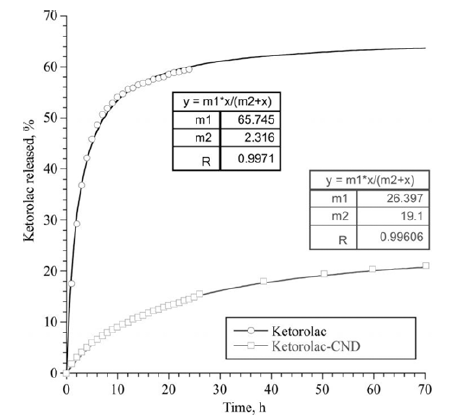
- Experiments on the long-term desorption carried out with DND/NSAID complexes showed a delayed effect of the nanoparticles on release of the drugs. The diffusion of the molecules of NSAID from the surface of the DND through the membrane was much slower than the diffusion of free drugs. This effect was observed for the both complexes of DND/NSAID. However, for the CND/ Ketorolac complex, the release of the drug was significantly slower than in the case of RND-COOH/Diclofenac. In this case, diffusion of free molecules of Diclofenac and Ketorolac occurred approximately at the same rate. Note that at the initial stage of exposure (time to reach half of the maximum value of the drug that passed through the membrane), the diffusion rate of the preparations within the complexes was several times less than this value for control solutions. Over time, the rate of diffusion of drugs not associated with nanoparticles tended to zero, while the rate of their diffusion from the surface of DND upon a small decrease remained almost constant.
- Figures 4-5 are graphs showing the change in the total number of drug molecules passed through the membrane with time. The experimental data are well approximated by a function based on the Michaelis-Menten equation, the coefficients of which are shown in the figures. Based on these dependencies, it is possible to calculate the required drug loading on nanoparticles taking into account the time.
- Results of our study showed that nanodiamonds posses properties required for an effective carrier in transdermal delivery of NSAID. Modified DND have an excellent colloidal stability and resist aggregation in aqueous solutions for long time, exhibit a high drug and cargo loading capacity, and display a sustained mechanism on the drug release thus able to provide its controlled dose to the skin. Moreover, a recent study showed the capability of nanodiamonds as a drug permeation enhancer to improve limited drug delivery through the stratum corneum with a high biocompatibility and reactive oxygen species scavenging effect [19].
Results and Discussion
Particle size distribution and z-potential of DND and DND-NSAID conjugates in aqueous suspensions

|
Total amount of Diclofenac passed through the membrane, as a percentage of the initial concentration.
Total amount of Ketorolac passed through the membrane, as a percentage of the initial concentration.
- Commercial detonation nanodiamonds were modified to reduce the amount of impurities and impart to the particles a high colloidal stability in water suspension. Modified DND retain colloidal stability after drying and can be re-dispersed in water without sonication. Physical adsorption was used to produce conjugates of the modified DND with nonsteroidal anti-inflammatory drugs Diclofenac and Ketorolac. The study of adsorption showed a high adsorption capacity of DND towards NSAID, which increases with the NSAID/DND mass ration and reaches up to 524 μg/mg. A pronounced relationship between adsorption and the charge of particles and NSAID was observed. However, a simple electrostatic model cannot explain all observed interactions between DND and NSAID. Experiments on desorption carried out with DND/NSAID complexes showed a sustained effect of the nanoparticles on drug release. Thus, the results of this study showed that detonation nanodiamonds are perspective materials to be used as a carrier in transdermal delivery of NSAID.
Conclusions
- 1. V. N. Mochalin, O. Shenderova, D. Ho and Y. Gogotsi: Nat. Nanotechnol., 7 (2012) 11..Article
- 2. R. G. Mendes, A. Bachmatiuk, B. Buchner, G. Cuniberti and M. H. Rummeli: J. Mater. Chem. B, 1 (2013) 401..Article
- 3. Nanodiamonds- Applications in Biology and Nanoscale Medicine, D. Ho (Ed.), Springer, New York (2010) 286..
- 4. K. M. El-Say: J. Appl. Pharm. Sci., 1 (2011) 29..
- 5. K. K. Liu, W. W. Zheng, C. C. Wang, Y. C. Chiu, C. L. Cheng, Y. S. Lo, C. Chen and J. I. Chao: Nanotechnology, 21 (2010) 315106..ArticlePubMed
- 6. V. N. Mochalin, A. Pentecost, X. M. Li, I. Neitzel, M. Nelson, C. Wei, T. He, F. Guo and Y. Gogotsi: Mol. Pharmaceutics, 10 (2013) 3728..ArticlePubMed
- 7. A. P. Puzyr, A. E. Burov, G. E. Selyutin, V. A. Voroshilov and V. S. Bondar: Tribology Transactions, 55 (2012) 149..Article
- 8. Detonation Nanodiamonds: Science and Applications, A. Vul and O. Shenderova (Ed.), Pan Stanford, Singapore (2014) 400..
- 9. A. P. Puzyr, A. E. Burov and V. S. Bondar: Fuller. Nanotub. Carbon Nanostruct., 23 (2015) 93..Article
- 10. T. Uchida, A. Hamano, N. Kawashima, S. Takeuchi: Electron. Commun. Jpn, Part III: Fundamental Electron. Sci., 90 (2007) 10..Article
- 11. I. Petrov, O. Shenderova, V. Grishko, V. Grichko, T. Tyler, G. Cunningham and G. McGuire.: Diamond Relat. Mater., 16 (2007) 2098..Article
- 12. K. Xu and Q. Xue: Diamond Relat. Mater., 16 (2007) 277..Article
- 13. Pragya and V. Rastogi: Int. J. Pharm. Sci. Res., 3 (2012) 2897..
- 14. M. N. Pastore, Y. N. Kalia, M. Horstmann and M. S. Roberts: Br. J. Pharmacol., 172 (2015) 2179..ArticlePubMedPMC
- 15. A. Krueger and D. Lang: Adv. Funct. Mater., 22 (2012) 890..Article
- 16. A. E. Aleksenskiy, E. D. Eydelman and A. Y. Vul: Nanosci. Nanotechnol. Lett., 3 (2011) 68..Article
- 17. N. Gibson, O. Shenderova, T. J. M. Luo, S. Mosenkov, V. Bondar, A. Puzyr, K. Purtov, Z. Fitzgerald and D.W. Brenner: Diamond Relat. Mater., 18 (2009) 620..Article
- 18. M. Aramesh, O. Shimoni, K. Ostrikov, S. Prawer and J. Cervenka: Nanoscale, 7 (2015) 5726..ArticlePubMed
- 19. D. G. Lim, K. H. Kim, E. Kang, S. H. Lim, J. Ricci, S. K. Sung, M. T. Kwon and S. H. Jeong: Int. J. Nanomed., 11(2016) 2381..PubMedPMC
Figure & Data
References
Citations

- Unveiling the trending paradigms of synthesis and theranostic biomedical potentials of nano-diamonds (NDs) - a state-of-the-art update
Sagnik Nag, Kedlaya Srikrishna H. Damodar, Swayambhik Mukherjee, Dinesh R. Rao, Ipsita Debnath, Sree Haryini, Sourav Mohanto, Mohammed Gulzar Ahmed, Vetriselvan Subramaniyan
Inorganic Chemistry Communications.2025; 177: 114313. CrossRef
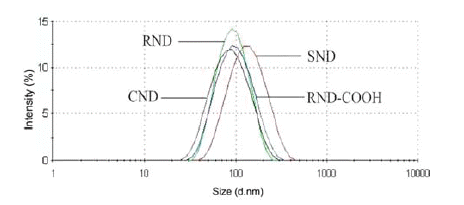

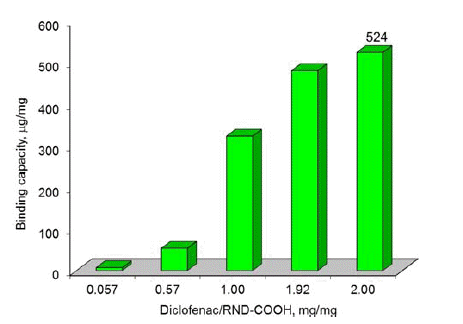

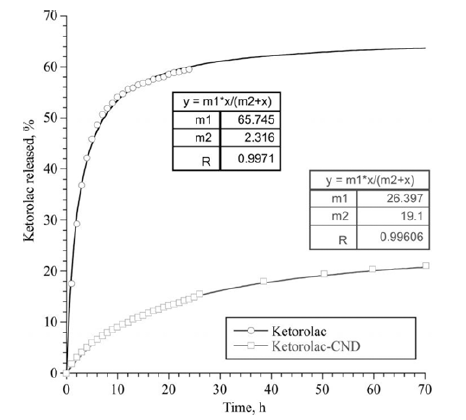
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
Table 1
Table 2
TOP
 KPMI
KPMI


 Cite this Article
Cite this Article





