Articles
- Page Path
- HOME > J Korean Powder Metall Inst > Volume 27(4); 2020 > Article
-
Article
- A Study on the Removal of Heavy Metal with Mg-Modified Zeolite
- Jei-Pil Wang*, Gyu-Cheol Kim, Min-Seok Go
-
Journal of Korean Powder Metallurgy Institute 2020;27(4):287-292.
DOI: https://doi.org/10.4150/KPMI.2020.27.4.287
Published online: July 31, 2020
Department of Metallurgical Engineering, Pukyong National University, Busan 48513, Republic of Korea
- *Corresponding Author: Jei-Pil Wang, TEL: +82-51-629-6741, FAX: +82-51-629-6742, E-mail: jpwang@pknu.ac.kr
- - Jei-Pil Wang: 교수, Gyu-Cheol Kim·Min-Seok Go: 학생
• Received: August 16, 2020 • Revised: August 22, 2020 • Accepted: August 26, 2020
© The Korean Powder Metallurgy Institute. All rights reserved.
- 1,812 Views
- 23 Download
- 2 Crossref
Abstract
- The subject of this study is a zeolite generated as a by-product of recycling LAS (lithium-aluminum-silicate) resources, a kind of glass and ceramic produced by induction. The zeolite by-product is modified into Mg-zeolite using Mg as a cation to absorb Pb, a heavy metal generated from water pollution caused by recent industrial wastewater. An ion-exchange method is used to carry out the modification process, from zeolite byproduct to Mg-zeolite, and simultaneously absorb the Pb in the heavy-metal solution (99.032 mg/L). It is found that the sodium zeolite in the raw material residue can be modified to magnesium zeolite by reacting it with a mixture solution at 1 M concentration for 24 h. As a result, it is found that the residual Pb (0.130 mg/L) in the heavy metal solution is shown to be absorbed by 99.86%, with successful formation of a Mg-modified zeolite.
- Since the water pollution becomes more serious among the pollution-related problems inevitably occurring in factories as a result of industrial development, many studies are currently being conducted. The main cause of environmental pollution due to the industrial wastewater from factories is the pollution by heavy metals, causing a very harmful effect on the human body. According to the report of the Ministry of Environment in 2019, the generation of industrial wastewater in Korea in 2017 was 4,921,000 m3/day [1]. The industrial wastewater requires meticulous control on account of its high concentration of pollution. The main substances contained in industrial wastewater are high concentration pollutants (i.e. heavy metals, organic compounds, mercury, acids, and alkaline detergents) [2]. Since the industrial wastewater contains a variety of harmful substances as above, a method suitable for hazardous substances must be applied for safe treatment, and the industrial wastewater must be discharged only when it satisfies the standards allowed for discharge.
- For the removal of heavy metals from substances contained in industrial wastewater, several methods have been suggested, including chemical, physical and biological methods [2]. Of them, many researchers are studying a method of using the zeolite as an adsorbent to remove heavy metal ions from the wastewater. Peric et al. (2004) conducted a study on removing zinc, copper and lead using natural zeolite as an adsorbent [3], and Kocaoba et al. (2007) conducted a study on removing cobalt, copper and nickel using natural zeolite as an absorbent [4].
- The zeolite is a generic term for the minerals in which alkaline earth metals are combined with anions formed by the combination of aluminum oxide and silicate oxide, referring to crystalline aluminum silicate minerals. The zeolite contain Al, Si, O, and H2O as essential components; and Na, Ca, K and Mg as selective components. The zeolite is a three-dimensional inorganic polymer in which silicon and aluminum are connected through 4 cross-linked oxygen, respectively, and at this time, as aluminum is combined with 4 oxygen, it has a negative charge. In order to offset this charge, cations such as selective components are needed. The inside of the micro-pores in the zeolite crystal is usually filled with water, but when heat is applied, water molecules inside the micro-pores escape into the atmosphere, creating an empty space [5]. At this time, small cations are free to enter and exit the cavity inside the empty space, and the zeolite modified with Mg2+ of many cations, which have larger spaces and volumes compared with other zeolite modified with cations such as Na+, K+, Ca2+, and Al3+, rapidly adsorbs organic-inorganic substances, removing even small particles [6, 7].
- In this study, the zeolite generated as a by product of LAS (Lithium-Aluminum-Silicate) circulating resource was modified with Mg cations to absorb and remove the Pb contained in the industrial wastewater on the effect to mixture concentration and reaction time.
1. Introduction
- 2.1 Experimental Materials
- To prepare the zeolite used as an adsorbent in this study, the recycled resources of the LAS (Lithium-Aluminum- Silicate) obtained from end-of-life of induction were pulverized and leached into the form of liquid. The zeolite contained in the solution was separated from the residue using a solvent extraction method. For the MgCl2∙ 6H2O (>98%) used for modification it to Mg-zeolite, a product from Junsei Chemical Co. Ltd (Tokyo, Japan) was used.
- 2.2 Experimental Method
- Mg-zeolite was prepared as follows. Before the experiment, the residue was dried at 100°C for 24 hours to remove moisture from it. To modify it into Mg2+ cationic type, a Mg mixture solution with a concentration of 0.25 M/0.5 M/1 M was prepared using MgCl2∙6H2O reagent and distilled water. 5 g of the residue dried in a 1 L beaker and 500 ml of the prepared Mg mixture solution of each concentration were stirred at 250 rpm for a certain period of time (12hours/2 4 hours) using a magnetic stirrer. After each reaction time (12h ours/24 hours) elapsed, the supernatant was discarded and it was replaced with Mg mixture solution of each concentration, and this process was repeated 3 times. It was then washed several times with distilled water, the moisture was removed by drying it at 100°C for 24 hours.
- For the wastewater solution to be used for the absorption of heavy metal, a wastewater solution (Pb 100 ppm) was prepared by diluting heavy metal standard reagent (Pb 1000 ppm, KANTO CHEMICAL CO. INC) solution and distilled water, and stirring it for 1 hour at 250 rpm using a magnetic stirrer.
- 2g of modified Mg-zeolite was added to 50 ml of the previously prepared wastewater solution (Pb 100 ppm) in a 500 ml beaker and stirred at 250 rpm for 24 hours using a magnetic stirrer to measure the heavy metal content in the solution.
- 2.3 Analysis Method
- XRD analyzer (Rigaku, UltimaIV), which is operated with 3 kw Cu-Kα X-Ray tube, was used to examine the phase transformation of the raw material residue and the modified zeolite, and FE-SEM (TESCAN, MIRA 3 LMH in-Beam Detector) was used to examine the size and shape of the crystal. In addition, ICP analyzer (Shimadzu, ICPS-1000IV) was used to measure the amount of Pb in the wastewater solution containing heavy metals (Pb).
2. Materials
2.2.1 Preparation of Mg-zeolite
Table 1
Preparation of Mg mixture solution (500ml)
| Mixture concentration | 0.25 M | 0.5 M | 1 M |
|---|---|---|---|
|
|
|||
| MgCl2 | 25.41 g | 50.83 g | 101.66 g |
2.2.2 Preparation of experimental wastewater solution
2.2.3 Adsorption of heavy metal (Pb)
- 3.1 Analysis of raw material residue
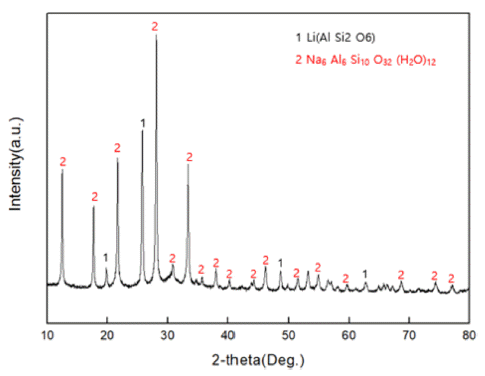
- Fig. 1 shows the phases of XRD analysis intended to confirm the existence of zeolite in the raw material residue. As a result of the measurement, it was confirmed that Na-zeolite, which is Na6Al6Si10O32(H2O)12, and β- phase spodumene of Li (AlSi2O) were existent.
- Fig. 2 shows the results of SEM analysis intended to examine the crystal shape of the zeolite in the raw material residue. It was confirmed that columnar crystals were existent in the form of Na zeolite with columnar crystals attached to it.
- 3.2 Modification of Mg-zeolite
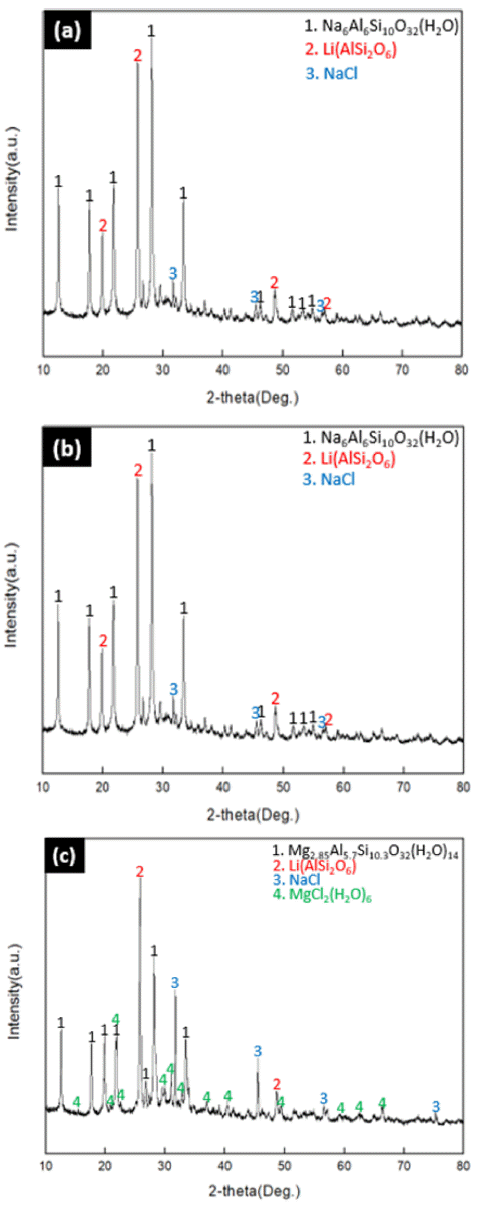
- In order to modify the Na-zeolite confirmed in 3.1 into Mg-zeolite, Mg mixture solution was reacted at a concentration of 0.25 M/0.5 M/1 M for 24 hours. The results of modification were confirmed by XRD analysis as shown in Fig. 3 below.
- When modified at the concentration of (0.25 M/0.5 M) in (a) and (b) of Fig 3., there was no change in the modification of the zeolite in raw material residue, and the formation of NaCl could be confirmed.
- However, when modified at the concentration of 1M in (c) of Fig 3, it has been modified into Mg zeolite [Mg2.85Al5.7Si10.3O32(H2O)14] and the excess MgCl2(H2O)6 remaining after reacting with the β-phase of Li (AlSi2O) and NaCl was existent.
- The reason why Mg zeolite was modified only in Fig. 3(c), it can be explained by factors that affect the catalytic properties of zeolite. The catalyst properties and adsorption properties of zeolite are closely related, and the modified synthetic zeolites with mainly large diameters have excellent performance. Therefore, Mg-zeolite modified with Mg has a larger cavity and higher spatial volume ratio than Zeolite modified with other cations (Na+, K+, Ca2+, Al3+), and has high surface activity. Accordingly, zeolite forms a structure with anions of AlOx and SiOx in structure. At this time, cations exist for charge offset, and these cations exist inside the pores, not inside the structure. When the ratio of SiO4/AlO4 i s 2 .5 or above, the resistance to acid and the cation exchange capacity decreases, whereas when the ratio decreases to 2 or below, the hydrophilicity and positive ion exchange characteristics increases. Therefore, in the case of (a) and (b) in Fig. 3 which are relatively at lower concentration than (c) in Fig. 3, the modification to Mg zeolite did not take place. [9] Table 2 shows chemical composition according to the concentration of mixture.
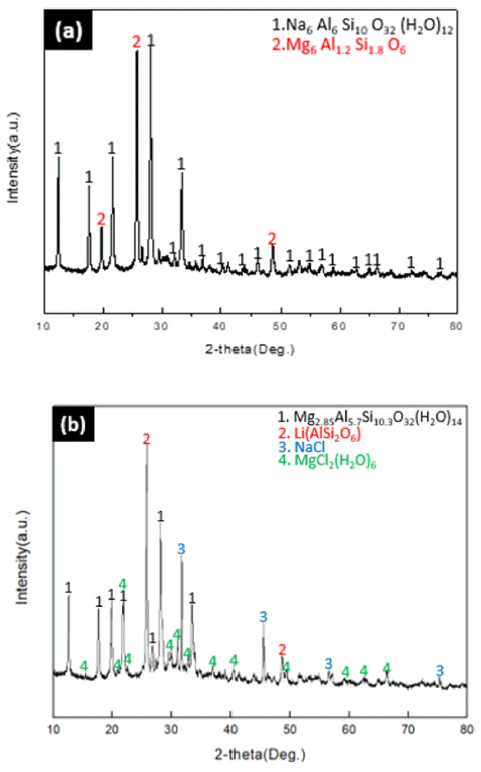
- In order to modify Na-zeolite into Mg-zeolite at a concentration of 1 M in which the modification reaction was confirmed in 3.2.1, a modification experiment was conducted by reaction time (12ho urs/24 hours). The modified phases were examined by XRD analysis, and the results are shown in Fig. 4.
- As shown in (a) of Fig. 4, when reacted for 12h ours, there was no change in the modification of Na-zeolite, but β-phase spodumene of Li (AlSi2O) was reacted with MgCl2∙6H2O, generating Mg6Al1.2Si1.8O6. However, the Mg-zeolite modification could be confirmed when reacted for 24 hours as shown in (b) of Fig. 4.
- In the case of 12-hour reaction, Mg2+ cations were changed by reaction with lithium of spodumene, not by with zeolite, but in the case of 24-hour reaction, reaction with zeolite took place first, followed by the reaction with Na and MgCl2 exchanged from zeolite took place, generating NaCl.
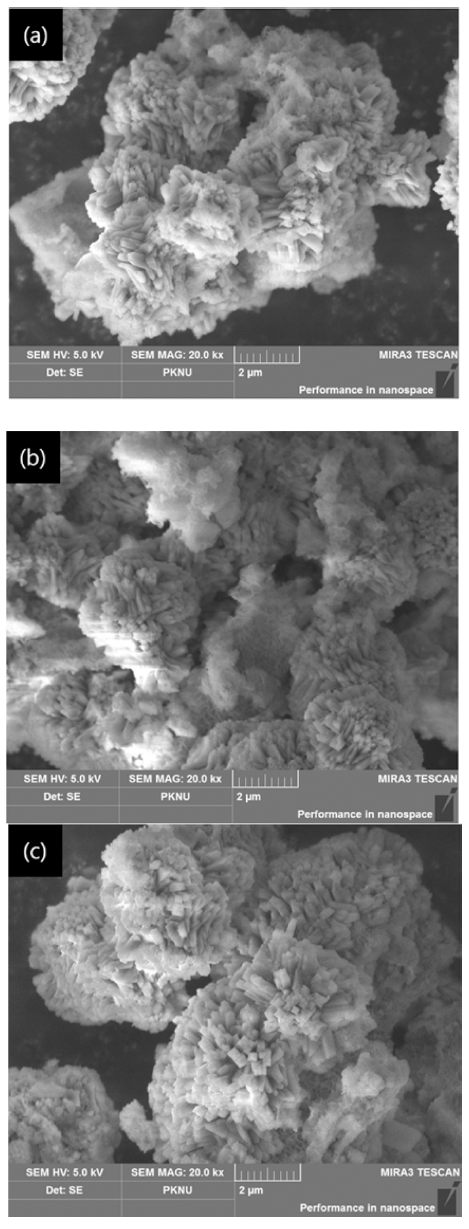
- As shown in (a) of Fig. 5 examined with FE-SEM, in the case of 12hou rs, the shape was entangled with each other compared to the raw material residue crystals, and a small amount of columnar crystals were existent, confirming that the crystals of zeolite were decomposed and reacted with spodumene, so they were entangled with each other. However, in the case of (b), more columnar crystals were existent than the raw material residue crystals, confirming that it was modified to Mg zeolite by ion exchange between Na and Mg.
- 3.3 Absorption test of heavy metal (Pb)
- 2g of Mg-modified zeolite was added to 50 ml of the wastewater solution prepared with a heavy metal standard reagent (Pb), and stirred for 24 hours at 250 rpm with a magnetic stirrer. After that, the content of Pb in the solution was analyzed by ICP analysis. As a result, the adsorption removal rate of 99.86% was confirmed as shown in Table 3. The zeolite is known to have better ion exchange capacity as the particle diameter is smaller. In the case of zeolite modified with Mg2+, it had the size of 4~5 Å, which is larger than the size of 3Å in the existing zeolite, due to changes in the structure of the existing zeolite, the nature and structural location of the cation, the content ratio of Si/Al, the presence of active metal elements, and the pH [8]. Therefore it is considered that with relatively excellent surface activity and large crystal pores, it showed high removal rate of 99.86% [10].
3. Results and Discussion
3.2.1 Modification by concentration of mixed solution
Table 2
Chemical compositions according to the concentration of the mixture
| wt.% | SiO2 | Al2O3 | MgO | Cl | Na2O |
|---|---|---|---|---|---|
|
|
|||||
| 0.25 M | 43.33 | 21.66 | 16.25 | 15.16 | 3.57 |
| 0.5 M | 51.61 | 24.73 | 11.82 | 6.45 | 5.37 |
| 1 M | 53.47 | 27.80 | 9.62 | 3.20 | 5.88 |
3.2.2 Modification by reaction time
- 4.1 Modification of Mg-zeolite
1) It was confirmed that the Na-zeolite in the raw material residue could be modified into Mg-zeolite by cation exchange with a MgCl2∙H2O mixture solution at 1M concentration.
2) It was found that the Na-zeolite in the raw material residue could be modified into Mg-zeolite by reacting it with mixture solution at 1M concentration for 24 hours.
- 4.2 Absorption of heavy metal (Pb)
4. Conclusion
-
Acknowledgements
- This study was supported by the BB21+ Project in 2020 and this material is based upon work supported by the Ministry of Trade, Industry & Energy (MOTIE, Korea) under Industrial Technology Innovation Program. No.20003877, ‘Development of eco-friendly electrochemical recycling system for production of high purity lithium and lithium compounds’
Acknowledgment
- 1. Ministry of Environment: Environmental Statistics Yearbook, (2019) 137. .
- 2. F. Fu and Q. Wang: J. Environ. Manage., 92 (2011) 407. .ArticlePubMed
- 3. J. Peric, M. Trgo and N. V. Mecvidovic: Water Res., 38 (2004) 1893. .ArticlePubMed
- 4. S. Kocaoba, Y. Orhan and T. Akyuz: Desalination, 214 (2007) 1. .Article
- 5. Z. Zhan, N. Ma, H. Yan, Y. Peng, Z. Wang and Y. Yan: J. Membr. Sci., 485 (2015) 94. .Article
- 6. K. Margeta, N. Z. Logar, M. Siljeg and A. Farkas: INTECH, Chapter5 (2013). .
- 7. E. Erdema, N. Karapinar and R. Donat: J. Colloid Interface Sci., 280 (2004) 309. .ArticlePubMed
- 8. P. Zhang,W. Ding, Y. Zhang, K. Dai and W. Liu: J. Chem. Pharm. Res., 6 (2014) 507. .
- 9. H. J. Choi: J. Korean Soc. Water Environ., 31 (2015) 392. .Article
- 10. G. B. Yun: Nano. Mater., 13 (2004) 2. .
Figure & Data
References
Citations
Citations to this article as recorded by 

- Penentuan Kualitas Kaolin sebagai Prekursor Sintesis Zeolit pada Kegiatan Praktikum
Rohmat Ismail, Manasye Erlangga, Ari Himawan, Givana Indah Nurul Afiah
Jurnal Pengelolaan Laboratorium Pendidikan.2025; 7(2): 81. CrossRef - Y-Type Zeolite Synthesized from an Illite Applied for Removal of Pb(II) and Cu(II) Ions from Aqueous Solution: Box-Behnken Design and Kinetics
Kinjal J. Shah, Jiacheng Yu, Ting Zhang, Zhaoyang You
Water.2023; 15(6): 1171. CrossRef
A Study on the Removal of Heavy Metal with Mg-Modified Zeolite
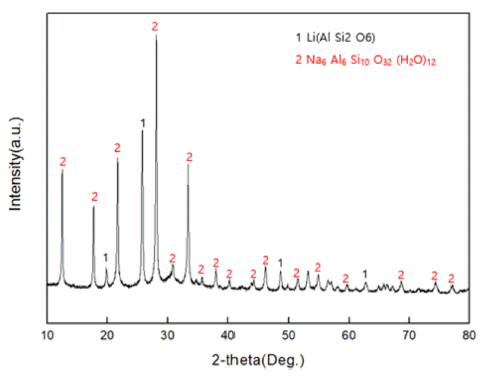

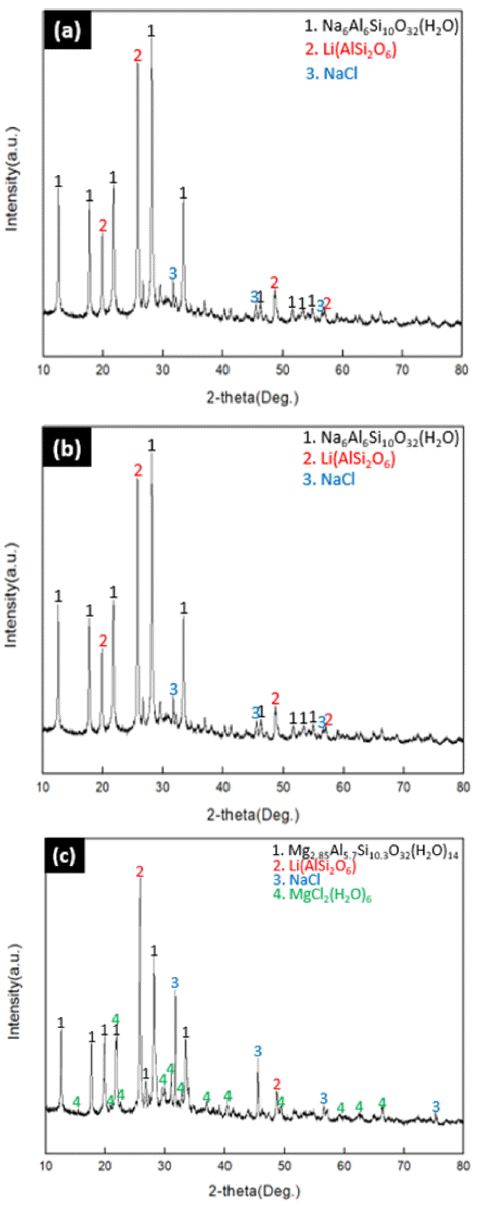
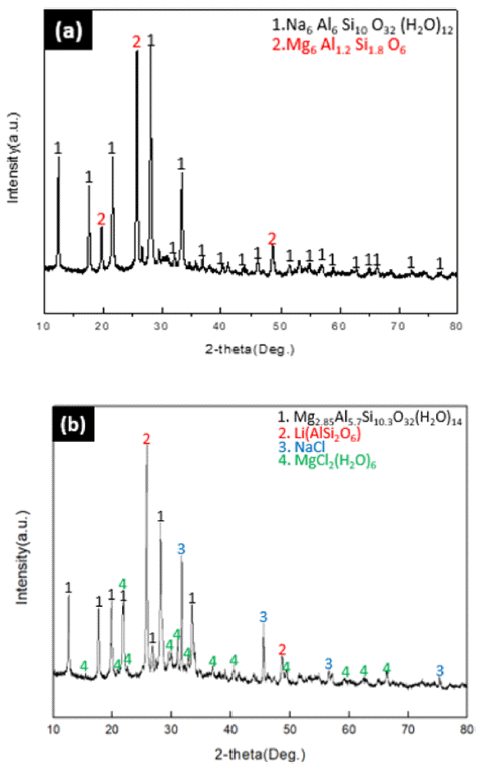
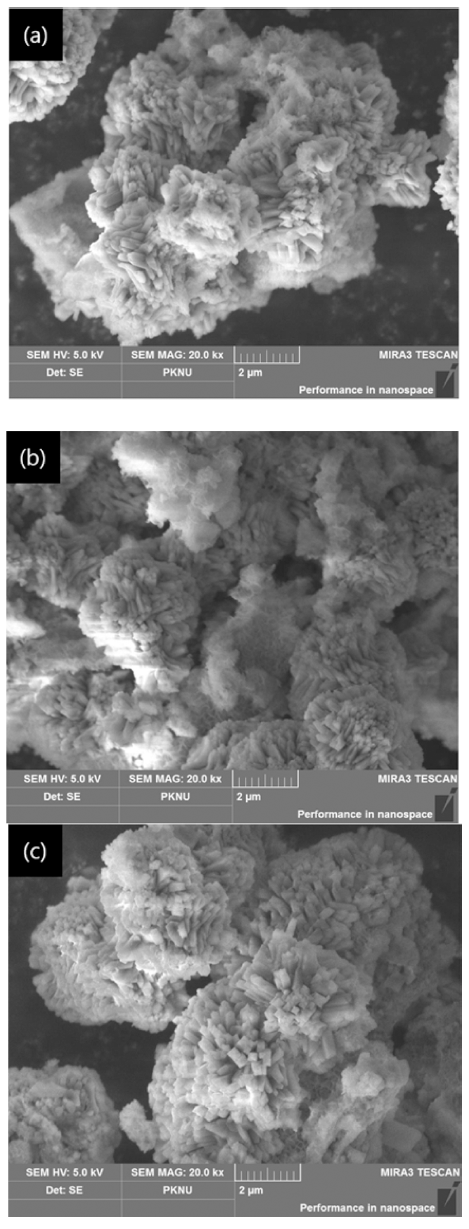
Fig. 1
XRD patterns of the zeolite in raw material residue.
Fig. 2
FE-SEM image of the zeolite in raw material residue.
Fig. 3
XRD results on the effect of mixture concentration. (a) 0.25 M (b) 0.5M (c) 1M.
Fig. 4
XRD patterns on the effect of reaction time (a) 12 hours (b) 24 hours.
Fig. 5
FE-SEM images by reaction time (a) raw specimen (b) 12 hours (c) 24 hours.
Fig. 1
Fig. 2
Fig. 3
Fig. 4
Fig. 5
A Study on the Removal of Heavy Metal with Mg-Modified Zeolite
| Mixture concentration | 0.25 M | 0.5 M | 1 M |
|---|---|---|---|
|
|
|||
| MgCl2 | 25.41 g | 50.83 g | 101.66 g |
| wt.% | SiO2 | Al2O3 | MgO | Cl | Na2O |
|---|---|---|---|---|---|
|
|
|||||
| 0.25 M | 43.33 | 21.66 | 16.25 | 15.16 | 3.57 |
| 0.5 M | 51.61 | 24.73 | 11.82 | 6.45 | 5.37 |
| 1 M | 53.47 | 27.80 | 9.62 | 3.20 | 5.88 |
| Pb in wastewater solution | Pb after absorption test | Removal rate |
|---|---|---|
|
|
||
| 99.032 mg/L | 0.130 mg/L | 99.86 (%) |
Table 1
Preparation of Mg mixture solution (500ml)
Table 2
Chemical compositions according to the concentration of the mixture
Table 3
Results of heavy metal (Pb) absorption
Table 1
Table 2
Table 3
TOP
 KPMI
KPMI


 Cite this Article
Cite this Article





