Articles
- Page Path
- HOME > J Powder Mater > Volume 31(6); 2024 > Article
-
Research Article
- Effect of Calcium Addition on the High-Temperature Recovery of Nd and Dy from Nd-Fe-B Scrap Using Mg-Based Extractants
- Hyoseop Kim*
-
Journal of Powder Materials 2024;31(6):493-499.
DOI: https://doi.org/10.4150/jpm.2024.00283
Published online: December 31, 2024
Korea Institute of Industrial Technology, 156, Gaetbeol-ro, Yeonsu-gu, Incheon 22099, Republic of Korea
- *Corresponding Author: Hyoseop Kim, TEL: +82-10-9477-6914, E-mail: hyoseop1231@kitech.re.kr
© The Korean Powder Metallurgy & Materials Institute
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,674 Views
- 17 Download
Abstract
- This study investigated whether calcium (Ca) addition improved the recovery of neodymium (Nd) and dysprosium (Dy) from Nd-Fe-B magnet scrap using magnesium (Mg)-based liquid metal extraction (LME). Traditional LME processes are limited to temperatures up to 850 °C due to oxidation issues, reducing the efficiency of rare earth element (REE) recovery, especially for Dy. By adding 10 wt.% Ca to Mg and increasing the processing temperature to 1,000 °C, we achieved nearly 100% Nd and approximately 38% Dy recovery, compared to 91% and 28%, respectively, with pure Mg at 850 °C. However, excessive Ca addition (20 wt.%) decreased the recovery efficiency due to the formation of stable intermetallic compounds. These results highlight the critical role of Ca in optimizing REE recycling from Nd-Fe-B magnet scrap.
- Rare earth elements (REEs), particularly neodymium (Nd) and dysprosium (Dy), are essential components in Nd-Fe-B permanent magnets due to their exceptional magnetic properties [1–4]. These magnets are crucial in technologies such as electric vehicles, wind turbines, medical devices, and electronic equipment [5]. The global demand for Nd-Fe-B magnets is increasing rapidly, with an estimated annual growth rate of 8–12%, driven by clean energy initiatives and the proliferation of high-tech devices [6, 7]. This surge intensifies pressure on REE supply chains, which are vulnerable due to geopolitical factors and the environmental impact of primary mining operations.
- Recycling Nd-Fe-B magnet scrap offers a sustainable alternative to primary mining, providing a secondary source of REEs while mitigating environmental degradation. However, efficient REE recovery from scrap poses significant challenges. Recycling methods are mainly hydrometallurgical or pyrometallurgical [8-11]. Hydrometallurgical techniques like acid leaching [9] and solvent extraction [11] are effective but involve complex processing steps, high operational costs, and generate hazardous waste requiring careful disposal. Pyrometallurgical approaches such as molten salt electrolysis [12, 13] and gas-phase extraction [14, 15] offer faster processing and simpler operation but are energy-intensive and may face scalability issues due to equipment and energy requirements.
- Liquid metal extraction (LME) has emerged as a promising pyrometallurgical method for recycling Nd-Fe-B scrap [16, 17]. In LME, molten metals like magnesium (Mg) selectively extract REEs based on their solubility and affinity compared to other elements like iron (Fe) and boron (B). This method benefits from operational simplicity and potential for continuous processing, making it attractive for industrial applications. However, while Mg effectively extracts light REEs (LREEs) like Nd, extraction of heavy REEs (HREEs) such as Dy remains inefficient due to Dy's lower solubility and tendency to form stable oxides, which hinder its recovery during the LME process [18-22].
- Enhancing Dy extraction is challenged by the formation of thermodynamically stable oxides like Dy2O3 at elevated temperatures [23], acting as diffusion barriers and impeding Dy dissolution into molten Mg. To mitigate oxidation, traditional LME operates below 850 °C, which is suboptimal for REE diffusion kinetics [24]. Recent studies have explored adding calcium (Ca) to molten Mg to address this issue [25, 26]. Ca can reduce REE oxides to their metallic forms, forming CaO as a byproduct that does not hinder the extraction process. This approach enables higher-temperature operations, improving the kinetics of REE extraction, particularly for HREEs like Dy. However, optimal conditions for Ca-assisted LME, including precise Ca content and the effects of high-temperature processing on Nd and Dy recovery, are not fully understood. Additionally, the impact of intermetallic compounds formed at higher Ca concentrations on REE solubility and extraction efficiency requires thorough investigation.
- This study aims to address these challenges by systematically investigating the effect of Ca addition and high-temperature processing on the extraction efficiencies of Nd and Dy from Nd-Fe-B magnet scrap using Mg-based extractants. By varying Ca content (0 wt.%, 10 wt.%, and 20 wt.%), processing temperatures (850 °C and 1,000 °C), and holding times (1 to 3 hours), we seek to determine the optimal conditions that maximize REE recovery without adverse effects from intermetallic compound formation. Understanding the thermodynamic and kinetic mechanisms by which Ca enhances the reduction of REE oxides and facilitates their diffusion into the molten alloy will contribute to optimizing the LME process for efficient recycling of Nd-Fe-B magnet scrap. Improving the recovery of HREEs like Dy is crucial due to their limited availability and significant role in enhancing the performance of Nd-Fe-B magnets, thereby supporting the advancement of clean energy technologies and electronic devices.
1. Introduction
- 2.1. Materials
- The Nd-Fe-B magnet scrap used in this study was sourced from industrial waste generated during the production of permanent magnets. The chemical composition of the scrap was determined using micro–X-ray fluorescence (μ-XRF) analysis, revealing approximately 23.84 wt.% neodymium (Nd), 8.57 wt.% dysprosium (Dy), 64.60 wt.% iron (Fe), 0.89 wt.% boron (B), along with minor amounts of cobalt (Co), aluminum (Al), and copper (Cu). The detailed composition is presented in Table 1.
- High-purity magnesium (Mg, ≥99.9%) and calcium (Ca, ≥99.9%) were used as extractant materials. Mg–Ca alloys were prepared with Ca concentrations of 0 wt.%, 10 wt.%, and 20 wt.% to evaluate the effect of Ca addition on REE extraction efficiency.
- 2.2. Sample Preparation
- The Nd-Fe-B magnet scrap was mechanically crushed using a jaw crusher to reduce the particle size, enhancing reaction kinetics during extraction. The crushed material was then sieved to obtain particles ranging from 1 mm to 2 mm. Approximately 200 g of the sieved magnet scrap was used for each experimental run.
- The prepared magnet scrap was placed into a stainless steel (SS) mesh container with a mesh size of 0.8 mm to retain the particles during the extraction process and allow efficient contact with the molten extractant.
- 2.3. Liquid Metal Extraction
- Extraction experiments were conducted using an induction furnace capable of reaching 1,100 °C. A crucible with an inner diameter of 100 mm and a height of 150 mm, featuring a sealed 10 mm bottom orifice, was used to hold the extractant metal. For each experiment, 200 g of Mg or Mg–Ca alloy (0 wt.%, 10 wt.%, or 20 wt.% Ca) was placed into the crucible. The magnet scrap, contained in a stainless steel (SS) mesh container, was positioned above the extractant metal within the crucible.
- Prior to heating, the assembly was placed inside a quartz chamber, evacuated to approximately 1 × 10⁻2 Pa to remove air and moisture, and purged with high-purity argon gas. This vacuum-purge cycle was repeated three times to minimize oxygen presence and reduce oxidation risk during heating.
- The furnace heated the crucible to target temperatures of 850 °C or 1,000 °C at a controlled rate of 10 °C/min. Temperature was monitored using a calibrated Type-K thermocouple near the crucible bottom. Once the target temperature was reached, the system was held isothermally for 1 to 3 hours, depending on the experimental conditions in Table 2.
- After the holding time, the system was cooled to approximately 800 °C to reduce the vapor pressures of Mg and Ca. The stopper rod sealing the crucible's bottom orifice was removed, allowing the molten alloy to flow into a preheated graphite mold beneath the crucible, which minimized thermal shock and facilitated alloy removal. During pouring, the stainless-steel mesh container holding the residual magnet scrap was gently agitated to ensure maximum drainage of the molten alloy and prevent entrapment of liquid metal among the scrap particles. The entire operation was conducted under continuous argon gas flow to maintain an inert atmosphere and prevent oxidation. After solidification in the mold under argon atmosphere, the alloy ingot and residual magnet scrap were cooled to room temperature and collected separately for analysis.
- 2.4. Sample Analysis
- The collected alloy ingots were sectioned using a diamond saw to obtain representative samples for analysis. The chemical compositions of both the alloy and the residual magnet scrap were determined using micro–X-ray fluorescence (μ-XRF).
- The extraction efficiency of Nd and Dy was calculated using the following equation:
- Where RREE is the extraction efficiency of the REE (Nd or Dy), wREEresidue is the mass fraction (wt.%) of the REE remaining in the residual magnet scrap after extraction, and wREEscrap is the mass fraction (wt.%) of the REE in the original magnet scrap before extraction.
2. Experimental Procedure
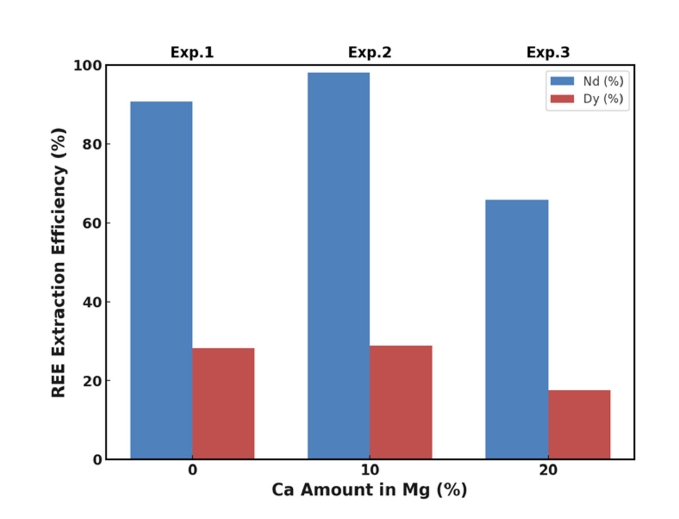
- 3.1. REE Extraction with Mg and Mg-Ca Alloys
- The chemical compositions of the alloys obtained from the liquid metal extraction (LME) experiments at 850 °C are summarized in Table 3.
- Fig. 1 illustrates the extraction efficiencies of Nd and Dy as a function of Ca addition at 850 °C. When pure magnesium (Mg) was used as the extractant (Exp. 1), the extraction efficiencies for Nd and Dy were approximately 91% and 28%, respectively. The high recovery of Nd aligns with Mg's known effectiveness in extracting light rare earth elements (LREEs) due to favorable thermodynamics and kinetics [18-22]. The lower recovery of Dy, a heavy rare earth element (HREE), is attributed to its higher thermodynamic stability in oxide form and lower diffusion rate in molten Mg [27].
- Adding 10 wt.% Ca to Mg (Exp. 2) significantly improved the extraction efficiency of Nd to nearly 100% and modestly increased Dy recovery to approximately 30%. This enhancement is primarily due to Ca's higher affinity for oxygen, enabling it to reduce stable REE oxides like Nd2O3 and Dy2O3 more effectively than Mg alone.
- The thermodynamic favorability of the reduction reactions can be quantified by the standard Gibbs free energy changes (ΔG°). At 850 °C, the reduction of Nd2O3 and Dy2O3 by Ca has ΔG° values of approximately –370 kJ/mol and –360 kJ/mol, respectively [18, 19]. These highly negative values indicate spontaneous reactions. In contrast, the corresponding reductions by Mg have less negative ΔG° values (approximately –180 kJ/mol for Nd2O3 and –170 kJ/mol for Dy2O3) [20], making them less favorable. The ability of Ca to reduce these oxides enhances the availability of metallic REEs for dissolution into the molten alloy.
- However, increasing the Ca content to 20 wt.% (Exp. 3) resulted in decreased extraction efficiencies for both Nd (approximately 66%) and Dy (approximately 18%). This decrease can be attributed to the formation of stable intermetallic compounds at higher Ca concentrations, such as Ca3Nd and Ca5Dy3, which have been reported in the literature [23, 25]. These compounds have low solubility in the Mg–Ca melt and tend to precipitate out, reducing the concentration of free REEs available for extraction.
- The formation of intermetallic phases not only consumes REEs but also alters the melt's properties, hindering mass transfer and diffusion processes. Previous studies have indicated that excessive Ca can lead to the formation of such intermetallic compounds, negatively impacting the extraction process [26]. These findings highlight the importance of optimizing Ca content to balance the benefits of oxide reduction against the drawbacks of intermetallic compound formation.
- 3.2. Effect of Temperature and Holding Time
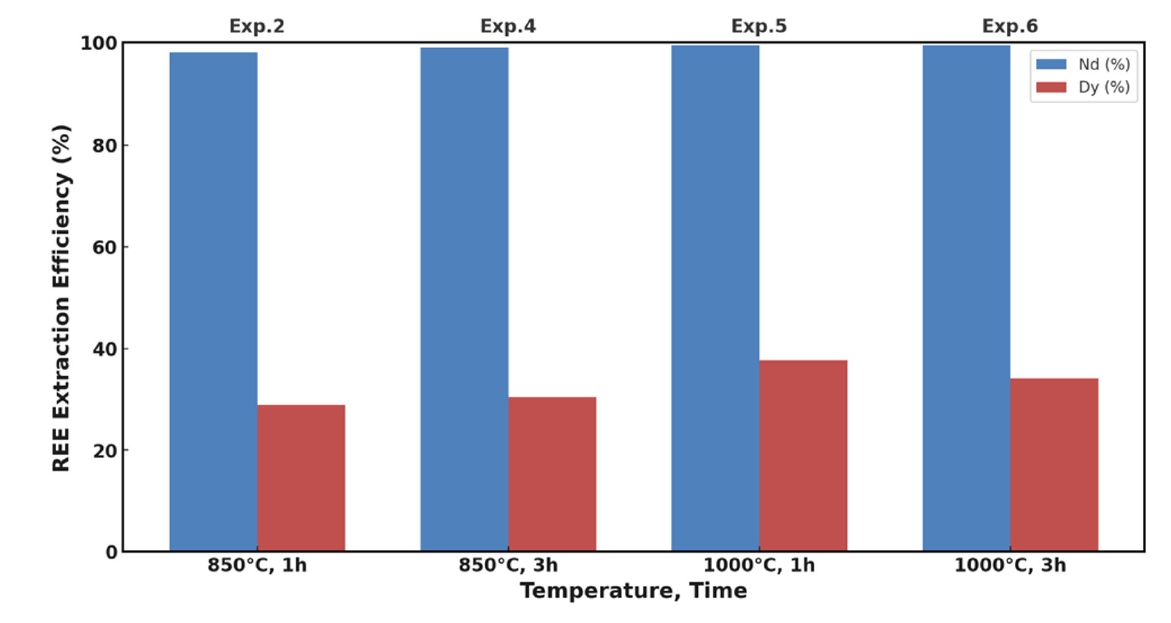
- The impact of temperature and holding time on the extraction efficiencies of Nd and Dy was evaluated using Mg–10 wt.% Ca alloy. The results are presented in Fig. 2. At 850 °C with a holding time of 1 hour (Exp. 2), Nd extraction efficiency was nearly 100%, while Dy recovery was approximately 30%. Extending the holding time to 3 hours at the same temperature (Exp. 4) had a minimal effect on Nd recovery but slightly improved Dy extraction to about 32%. This suggests that Nd extraction is kinetically fast once the oxide barrier is reduced, whereas Dy extraction is diffusion-limited.
- Raising the temperature to 1,000 °C significantly enhanced Dy extraction efficiency to approximately 38% after 1 hour (Exp. 5). However, extending the holding time to 3 hours at 1,000 °C (Exp. 6) resulted in a decrease in Dy recovery to about 35%, indicating that prolonged exposure at high temperatures may adversely affect Dy extraction.
- The decrease in Dy recovery with extended holding time can be attributed to several factors. One possible explanation is the formation of stable intermetallic compounds between Dy and Ca or Mg, such as Ca5Dy3 or Mg3Dy, which have low solubility in the molten alloy and tend to precipitate out, reducing the amount of free Dy available for extraction [23, 25]. Additionally, prolonged high-temperature exposure can increase the vaporization of Mg and Ca due to their high vapor pressures, altering the composition of the extractant and reducing its effectiveness [26]. Despite maintaining an inert atmosphere, trace amounts of oxygen could lead to re-oxidation of Dy over extended periods, forming Dy2O3 and diminishing extraction efficiency.
- Despite the thermodynamic favorability of Dy reduction at higher temperatures, these factors can counteract the benefits, leading to decreased Dy extraction with prolonged holding times. Dy extraction remains lower than Nd due to kinetic limitations and its higher tendency to form stable compounds. Dy has a higher melting point (1,412 °C) and a smaller atomic radius, contributing to slower diffusion rates in the molten alloy [27]. Additionally, Dy2O3 is more thermodynamically stable than Nd2O3, requiring higher activation energy for reduction.
- 3.3. Mechanism of REE Oxide Reduction by Calcium
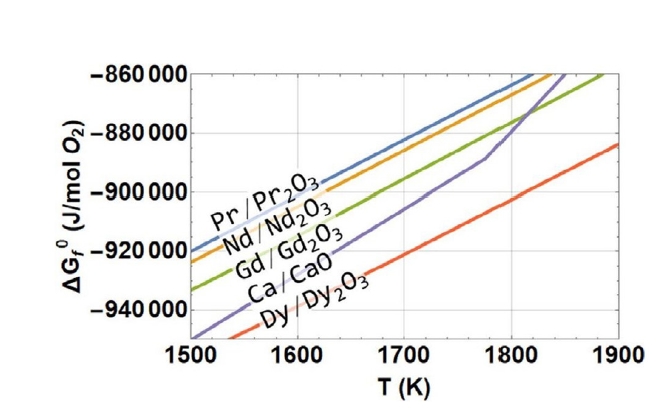
- Ellingham diagrams (Fig. 3) were used to compare the stability of oxides and the tendency of different elements to form oxides. Ca has a more negative standard free energy of formation for its oxide (CaO) compared to Nd2O3 and Dy2O3, indicating that Ca can effectively reduce these REE oxides. The reduction reactions are thermodynamically favorable across the temperature range studied, with the driving force increasing at higher temperatures.
- The reduction reactions can be represented as:
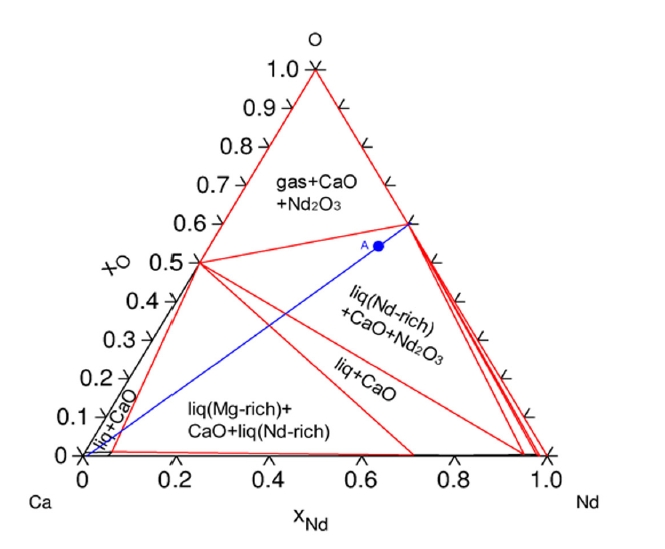
- The Ca–Nd–O ternary phase diagrams at 1,000 °C (Fig. 4) illustrate the phase equilibria and confirm the formation of CaO and metallic REEs upon reduction of REE oxides by Ca. These diagrams also show that excessive Ca can lead to the formation of intermetallic compounds (e.g., Ca3Nd, CaNd, Ca5Dy3), which correspond with the decreased extraction efficiencies observed at higher Ca concentrations.
- The mechanism of REE oxide reduction by Ca involves the following steps:
- (1) Reduction of REE Oxides: Calcium reduces Nd2O3 and Dy2O3 to metals, forming CaO.
- (2) Dissolution into Molten Alloy: The reduced REEs dissolve into the molten Mg–Ca alloy.
- (3) Formation of CaO: CaO forms and may precipitate from the melt due to its high melting point.
- The overall reaction can be simplified as:
- Where REE represents Nd or Dy.
- The removal of oxygen enhances the solubility of REEs in the molten alloy. However, Dy's propensity to form stable intermetallic compounds at higher Ca concentrations limits its solubility. This underscores the necessity of optimizing Ca content to prevent excessive formation of such compounds.
- 3.4. Comparison with Previous Studies
- The findings of this study demonstrate an improved Dy extraction efficiency (~38%) compared to previous reports, which typically achieved Dy recoveries of 20–30% using pure Mg at lower temperatures. The use of Ca as a reducing agent and the increase in processing temperature to 1,000 °C effectively enhanced the reduction of Dy2O3, facilitating greater Dy recovery. Furthermore, identifying an optimal Ca content (~10 wt.%) aligns with the need to balance oxide reduction benefits against the formation of undesirable intermetallic compounds. This balance was not thoroughly explored in prior studies, highlighting the contribution of this research to advancing the understanding of Ca-assisted LME processes.
- Incorporating Ca into the LME process at controlled concentrations can significantly enhance REE recovery from Nd-Fe-B magnet scrap, particularly for HREEs like Dy. Operating at higher temperatures (up to 1,000 °C) is feasible with Ca addition, as it mitigates oxidation concerns. However, careful control of Ca content is essential to prevent the formation of intermetallic compounds that reduce extraction efficiency.
- 3.5. Implications and Future Work
- This study highlights the pivotal role of Ca in overcoming oxidation barriers and optimizing the LME process for REE recycling. The optimized addition of Ca at around 10 wt.% to Mg-based extractants, combined with high-temperature processing at 1,000 °C, significantly enhances the recovery of both Nd and Dy from Nd-Fe-B magnet scrap. These findings provide valuable insights into the thermodynamic and kinetic factors governing the LME process and emphasize the importance of precise Ca content control.
- Future research should focus on further improving Dy recovery by exploring alternative reducing agents or alloying elements that facilitate Dy extraction without forming undesirable intermetallic compounds. Additionally, optimizing process parameters such as holding time, temperature profiles, and atmosphere control could lead to even more efficient extraction processes. Assessing the scalability of this method for industrial applications is also crucial for advancing sustainable recycling practices for critical rare earth elements.
3. Results and Discussion
- Adding 10 wt.% calcium (Ca) to magnesium (Mg)-based extractants significantly enhanced the extraction of neodymium (Nd) and dysprosium (Dy) from Nd-Fe-B magnet scrap at elevated temperatures. At 1,000 °C, Nd extraction efficiency reached nearly 100%, and Dy recovery improved to approximately 38%. This improvement is due to Ca's strong affinity for oxygen, effectively reducing stable REE oxides like Nd2O3 and Dy2O3, which facilitates the release and diffusion of metallic REEs into the molten Mg–Ca alloy.
- However, increasing Ca content to 20 wt.% decreased extraction efficiencies for both Nd and Dy because of the formation of stable intermetallic compounds like Ca3Nd and Ca5Dy3. These compounds have low solubility in the Mg–Ca melt, reducing the availability of free REEs. Optimizing Ca content is therefore crucial to balance oxide reduction benefits against intermetallic compound formation.
- Operating at higher temperatures enhanced reaction kinetics and thermodynamic favorability, further improving Dy recovery. However, prolonged holding times at high temperatures slightly decreased Dy extraction efficiency, likely due to additional intermetallic formation, increased vaporization losses of Mg and Ca, and potential re-oxidation of Dy.
- This research advances the understanding of Ca-assisted liquid metal extraction (LME) for REE recycling. By optimizing Ca content and processing conditions, oxidation barriers can be overcome, enhancing the recovery of both light and heavy REEs from Nd-Fe-B scrap. These findings have significant implications for developing efficient, scalable recycling methods, reducing reliance on primary REE mining, and supporting sustainable supply chains.
- Future work should focus on further enhancing Dy recovery by exploring alternative reducing agents or alloying elements that prevent undesirable intermetallic compounds while maintaining effective oxide reduction. Refining process parameters and assessing the scalability and economic feasibility of this method are essential for potential industrial application.
4. Conclusion
-
Funding
This work was supported by grants from the Korea Institute of Industrial Technology (KITECH) under the “Manufacturing Innovation Support Program” (KITECH JH-24-0008, KITECH JH-24-0013).
-
Conflict of Interest Declaration
The authors have no conflicts of interest to declare.
-
Data Availability Statement
All dataset files used in this study are already provided in the manuscript.
-
Author Information and Contribution
Hyoseop Kim: Researcher; conceptualization, writing–original draft, writing–review & editing, funding acquisition, supervision.
-
Acknowledgement
None.
Article information
| Elements | Nd | Dy | Fe | B | Co | Al | Cu |
|---|---|---|---|---|---|---|---|
| Concentration (wt.%) | 23.84 | 8.57 | 64.60 | 0.89 | 1.92 | 0.06 | 0.12 |
- 1. Z. Hua, J. Wang, L. Wang, Z. Zhao, X. Li and Y. Xiao: ACS Sustainable Chem. Eng., 2 (2014) 2536.Article
- 2. M. Sagawa, S. Fujimura, N. Togawa, H. Yamamoto and Y. Matsuura: J. Appl. Phys., 55 (1984) 2083.ArticlePDF
- 3. K. Binnemans, P. T. Jones, B. Blanpain and T. V. Gerven: J. Cleaner Prod., 51 (2014) 1.Article
- 4. J. J. Croat, J. F. Herbst, R. W. Lee and F. E. Pinkerton: J. Appl. Phys., 55 (1984) 2078.ArticlePDF
- 5. H.-S. Yoon, C.-J. Kim, K. W. Chung, S.-J. Lee and A.-R. Joe: Korean J. Chem. Eng., 31 (2014) 706.ArticlePDF
- 6. D. Dupont and K. Binnemans: Green Chem., 17 (2015) 2150.Article
- 7. O. Takeda, T. H. Okabe and Y. Umetsu: J. Alloys Compd., 392 (2005) 206.Article
- 8. J. W. Lyman and G. R. Palmer: High Temp Mater Process, 11 (1993) 175.
- 9. X. Sun and K. E. Waters: ACS Sustainable Chem. Eng., 2 (2014) 1910.Article
- 10. M.-S. Lee, J.-Y. Lee, J.-S. Kim and G.-S. Lee: Sep. Purif. Technol., 46 (2005) 72.Article
- 11. T. Itakura, R. Sasai and H. Itoh: J. Alloys Compd., 408-412 (2006) 1382.Article
- 12. M. Itoh, K. Miura and K.-I. Machida: Chem. Lett., 37 (2008) 372.ArticlePDF
- 13. T. Uda: Mater. Trans., 43 (2002) 55.Article
- 14. K. Murase, K. Machida and G. Adachi: J. Alloys Compd., 217 (1995) 218.Article
- 15. Y. Mochizuki, N. Tsubouchi and K. Sugawara: ACS Sustainable Chem. Eng., 1 (2013) 655.Article
- 16. T. W. Ellis and F. A. Schmidt: Ames, Iowa, US Patent, 5,437,709, (1995.
- 17. Y. Xu, L. Chumbley and F. Laabs: J. Mater. Res., 15 (2000) 2296.Article
- 18. T. H. Okabe, O. Takeda, K. Fukuda and Y. Umetsu: Mater. Trans., 44 (2003) 798.Article
- 19. O. Takeda, T. H. Okabe and Y. Umetsu: J. Alloys Compd., 408-412 (2006) 387.Article
- 20. H. J. Chae, Y. D. Kim, B. S. Kim, J. G. Kim and T.-S. Kim: J. Alloys Compd., 586 (2014) S143.Article
- 21. H. W. Na, Y. H. Kim, H. T. Son, I. H. Jung, H. S. Choi and T. B. Kim: Curr. Nanosci., 10 (2014) 128.Article
- 22. M. Sun, X. Hu, L. Peng, P. Fu, W. Ding and Y. Peng: J. Mater. Process. Technol., 218 (2015) 57.Article
- 23. S.-W. Nam, S.-M. Park, D.-H. Kim and T.-S. Kim: Metals and Materials International, 27 (2020) 538.ArticlePDF
- 24. S.-W. Nam, S.-M. Park, M. Z. Rasheed, M.-S. Song, D.-H. Kim and T.-S. Kim: Metals, 11 (2021) 1345.Article
- 25. R. Schmid-Fetzer, A. Kozlov, B. Wiese, C. L. Mendis, D. Tolnai, K. U. Kainer and N. Hort, Magnesium Technology, 2016, Springer, Cham (2016) 67.
- 26. S.-H. Ha, J.-K. Lee and S. K. Kim: Mater. Trans., 49 (2008) 1081.Article
- 27. M. Firdaus, M. A. Rhamdhani, Y. Durandet, W. J. Rankin and K. McGregor: J. Sustain. Metall., 2 (2016) 276.ArticlePDF
References
Figure & Data
References
Citations

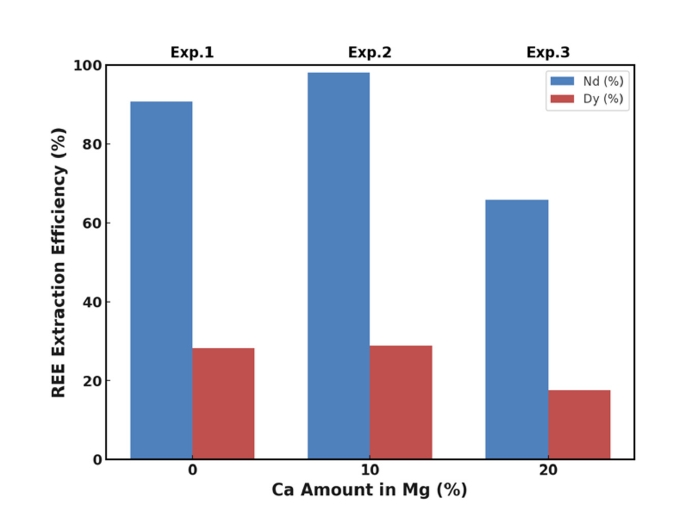
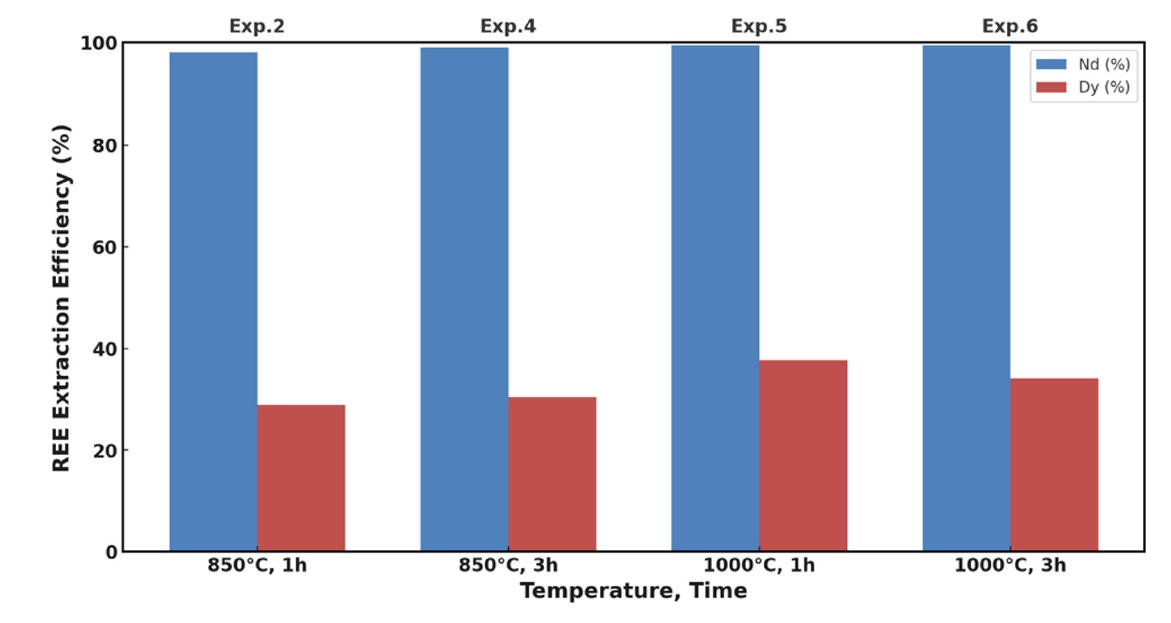
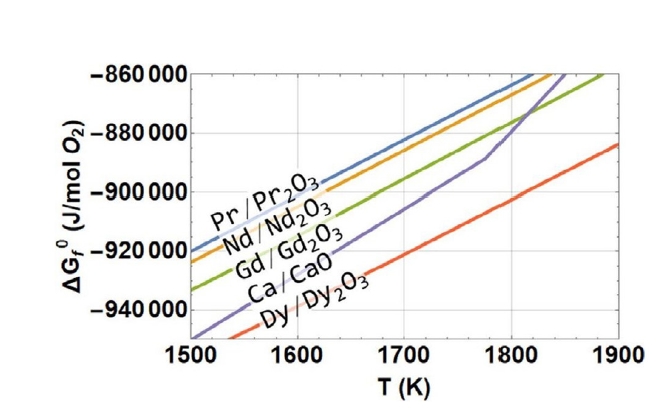
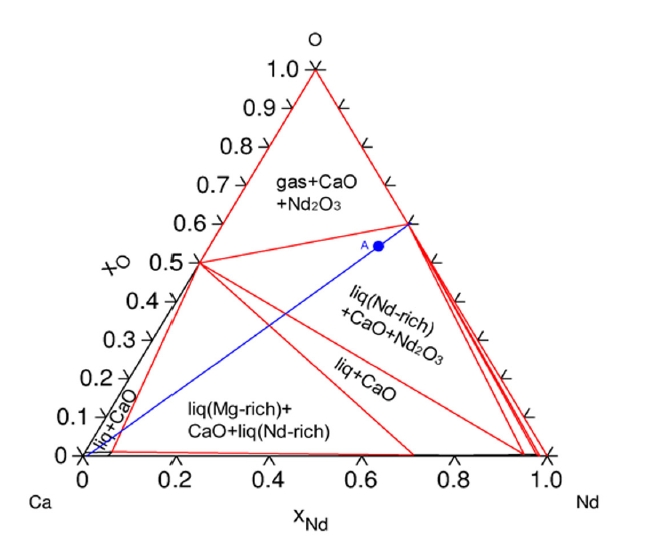
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
| Elements | Nd | Dy | Fe | B | Co | Al | Cu |
|---|---|---|---|---|---|---|---|
| Concentration (wt.%) | 23.84 | 8.57 | 64.60 | 0.89 | 1.92 | 0.06 | 0.12 |
| Experiment Number | Temperature (˚C) | Holding Time (h) | NdFeB Scrap (g) | Extractant |
|
|---|---|---|---|---|---|
| Mg (g) | Ca (g) | ||||
| Exp. 1 | 850 | 1 | 200 | 200 | - |
| Exp. 2 | 850 | 1 | 200 | 180 | 20 |
| Exp. 3 | 850 | 1 | 200 | 160 | 40 |
| Exp. 4 | 850 | 3 | 200 | 180 | 20 |
| Exp. 5 | 1,000 | 1 | 200 | 180 | 20 |
| Exp. 6 | 1,000 | 3 | 200 | 180 | 20 |
| Experiment Number | Chemical composition (wt.%) |
Mg to Ca Ratio | ||||
|---|---|---|---|---|---|---|
| Nd | Dy | Mg | Ca | Fe | ||
| Exp. 1 | 16.05 | 1.84 | 82.09 | - | 0.02 | - |
| Exp. 2 | 17.95 | 1.88 | 70.16 | 10.00 | 0.00 | 7.02 |
| Exp. 3 | 10.11 | 1.15 | 66.56 | 22.16 | 0.03 | 3.00 |
| Exp. 4 | 20.08 | 1.98 | 67.44 | 10.51 | 0.00 | 6.42 |
| Exp. 5 | 21.11 | 2.45 | 64.52 | 11.73 | 0.19 | 5.50 |
| Exp. 6 | 24.28 | 2.22 | 60.11 | 13.21 | 0.18 | 4.55 |
Table 1.
Table 2.
Table 3.
TOP
 KPMI
KPMI

 ePub Link
ePub Link Cite this Article
Cite this Article




