Articles
- Page Path
- HOME > J Powder Mater > Volume 31(6); 2024 > Article
-
Critical Review
- Advances in Powder Metallurgy for High-Entropy Alloys
- Sheetal Kumar Dewangan1, Cheenepalli Nagarjuna1, Hansung Lee1, K. Raja Rao2, Man Mohan1,3, Reliance Jain1, Byungmin Ahn1,4,*
-
Journal of Powder Materials 2024;31(6):480-492.
DOI: https://doi.org/10.4150/jpm.2024.00297
Published online: December 31, 2024
1Department of Materials Science and Engineering, Ajou University, Suwon 16499, Republic of Korea
2Department of Mechanical Engineering, Mandsaur University, Mandsau, 458002, India
3Department of Mechanical Engineering, Rungta College of Engineering and Technology, Bhilai 490024, India
4Department of Energy Systems Research, Ajou University, Suwon 16499, Republic of Korea
- *Corresponding Author: Byungmin Ahn TEL: +82-31-219-3531, FAX: +82-31-219-1613, E-mail: byungmin@ajou.ac.kr
© The Korean Powder Metallurgy & Materials Institute
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,762 Views
- 119 Download
- 2 Crossref
Abstract
- High-entropy alloys (HEAs) represent a revolutionary class of materials characterized by their multi-principal element compositions and exceptional mechanical properties. Powder metallurgy, a versatile and cost-effective manufacturing process, offers significant advantages for the development of HEAs, including precise control over their composition, microstructure, and mechanical properties. This review explores innovative approaches integrating powder metallurgy techniques in the synthesis and optimization of HEAs. Key advances in powder production, sintering methods, and additive manufacturing are examined, highlighting their roles in improving the performance, advancement, and applicability of HEAs. The review also discusses the mechanical properties, potential industrial applications, and future trends in the field, providing a comprehensive overview of the current state and future prospects of HEA development using powder metallurgy.
- High-entropy alloys (HEAs) have emerged as a groundbreaking class of materials with unique properties and diverse potential applications. Unlike traditional alloys, which typically consist of one or two principal elements, HEAs are composed of multiple principal elements (usually five or more) in near-equiatomic ratios [1–4]. This complex composition results in a high configurational entropy, which stabilizes the formation of simple solid solution phases and imparts exceptional mechanical and physical properties. HEAs are known for their superior strength [5], excellent wear [6] and corrosion resistance [7], and remarkable high-temperature stability [8], making them highly attractive for advanced engineering applications in industries such as aerospace, automotive, and energy [9, 10].
- Despite their promising attributes, the development and commercialization of HEAs face significant challenges, primarily due to the difficulties associated with their fabrication. Conventional manufacturing techniques often fall short in producing HEAs with consistent quality and desirable properties. This is where powder metallurgy (PM) techniques come into play. PM offers a versatile and cost-effective approach to producing HEAs, allowing for precise control over composition and microstructure. The inherent advantages of PM, such as the ability to produce fine and uniform powders, complex shapes, and high-performance materials, make it an ideal method for HEA fabrication [11–13]. In addition, HEAs typically consist of multiple elements in near-equiatomic proportions, leading to a high degree of atomic mixing. This randomness in atomic arrangement increases configurational entropy, which stabilizes the solid solution phase because the system prefers higher entropy for thermodynamic stability.
- Configurational entropy discourages the formation of intermetallic compounds because these ordered structures have lower entropy. Intermetallic compounds are more rigid, with specific atomic arrangements, which reduce the system's overall entropy. In contrast, solid solutions allow for more atomic disorder, leading to higher entropy and thus more stability at elevated temperatures. This stabilization mechanism contributes to the unique properties of HEAs, such as improved mechanical strength, corrosion resistance, and thermal stability, making them superior for various high-performance applications.
- When comparing PM with conventional alloy production methods, several distinct differences and advantages emerge. Conventional methods typically involve significant material waste due to machining and cutting processes, whereas PM offers near-net shape capabilities, resulting in minimal material waste. Conventional methods often require multiple steps and extensive machining to achieve complex geometries, while PM can produce intricate shapes directly from the powder, reducing the need for additional machining [14, 15]. Microstructural control is another area where PM excels; conventional methods may result in inhomogeneous microstructures due to the melting and solidification processes, whereas PM provides better control and uniformity through controlled powder production and sintering processes. In terms of processing temperature and energy consumption, conventional methods typically involve high temperatures for melting and casting, leading to higher energy consumption, whereas PM often operates at lower temperatures, particularly in sintering and consolidation steps, resulting in energy savings [16]. Mechanical properties are also enhanced in PM, which can achieve superior properties through uniform microstructures, enhanced diffusion bonding, and advanced consolidation techniques like hot isostatic pressing (HIP) and spark plasma sintering (SPS) [15, 17–19]. PM offers greater flexibility in alloy design, enabling the development of novel alloys, including HEAs, with unique properties, whereas conventional methods are limited in the range of compositions that can be processed due to melting point differences and segregation issues. While conventional methods are generally more cost-effective for high-volume production of standard alloys, PM can be more cost-effective for producing specialized, high-performance alloys and components, especially in lower volumes or for complex shapes. In conclusion, powder metallurgy provides a versatile and efficient approach to alloy development, offering significant advantages in terms of material utilization, complexity of shapes, microstructural control, and energy consumption. These benefits make PM an attractive option for developing advanced materials like high-entropy alloys, which require precise control over composition and properties [11, 12, 20, 21].
- In advancement, machine learning (ML) is transforming powder metallurgy in the production of HEAs by accelerating the discovery, optimization, and processing of these complex materials with improved material efficiency. In HEAs, where numerous elements combine in near-equiatomic proportions, the vast design space makes traditional trial-and-error approaches time-consuming and cost-prohibitive. ML algorithms can efficiently analyze and predict optimal compositions, powder processing parameters, and sintering conditions by learning from extensive datasets on composition, microstructure, and performance metrics. For instance, ML models can predict how variations in milling speed, time, or temperature will influence microstructure homogeneity, grain size, and phase stability. Additionally, ML can aid in identifying ideal parameters for high-energy ball milling and sintering to maximize chemical uniformity and mechanical properties. By enhancing process control and material characterization, ML in powder metallurgy improves the efficiency and precision of HEA development, leading to faster innovations and superior performance of these advanced alloys [22, 23].
- Here, presenting a comprehensive overview of advancements in powder production, sintering methods, and additive manufacturing. Unlike previous reviews, it emphasizes PM’s unique ability to finely control HEA composition and microstructure, which is essential for tailoring mechanical properties and enhancing performance. Additionally, this review extends beyond technical discussions to address current and emerging industrial applications for PM-processed HEAs, providing valuable insights into future trends and the evolving landscape of HEA research. By integrating detailed analyses of PM process innovations and their impact on HEA mechanical properties, this review serves as a critical resource for researchers and industry professionals seeking to leverage PM techniques for specific HEA applications. Thus, this paper aims to explore the revolutionary impact of powder metallurgy techniques on the development of high-entropy alloys. It will discuss the various PM processes, including atomization, mechanical alloying, and advanced sintering techniques, and how they contribute to the optimization of HEAs. Furthermore, the paper will delve into the applications of HEAs produced through PM, highlighting their superior properties and potential industrial uses. By examining the latest advancements and future trends in this field, this review seeks to provide a comprehensive understanding of how PM is transforming the landscape of high-entropy alloy development and paving the way for next-generation materials.
1. Introduction
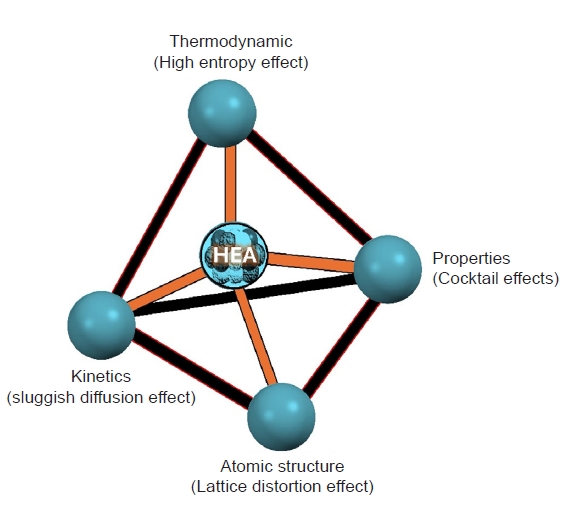
- HEAs represent a novel class of materials characterized by their unique composition and exceptional properties. Unlike traditional alloys, which typically consist of one or two principal elements, HEAs are composed of five or more elements in near-equiatomic proportions [24–26]. This unconventional approach to alloy design leads to a variety of beneficial characteristics, making HEAs a subject of intense research and interest in materials science. A brief fundamental has been present in Fig. 1.
- HEAs were first introduced in the early 2000s as researchers sought to explore the untapped potential of multi-component systems. Unlike traditional alloy design, which focuses on a principal element with minor additions. Initial studies revealed that HEAs could form stable, single-phase structures despite their complex compositions, attributed to the high entropy of mixing, which stabilizes disordered solid solutions over intermetallic compounds. The field of HEAs has rapidly expanded, with ongoing research into various element combinations to tailor properties for specific applications. Advances in computational methods and high-throughput experimental techniques have accelerated HEA development, promising significant impacts across industries such as aerospace, automotive, biomedical, and energy. Despite developmental challenges, the unique properties of HEAs continue to drive innovation and interest in this groundbreaking field of alloy design [28–30].
- 2.1. Constituents of Elements and Structure
- HEAs are a novel class of materials defined by their unique compositional complexity. Unlike conventional alloys, which typically consist of one or two primary elements with minor additions, HEAs are composed of five or more principal elements in near-equiatomic ratios. This high configurational entropy leads to the stabilization of simple solid solution phases rather than complex intermetallic compounds.
- I. Multi-Principal Element Composition: HEAs are designed with multiple elements, each contributing significantly to the overall composition. This multi-element approach increases the entropy of mixing, which promotes the formation of simple structures like face-centered cubic (FCC), body-centered cubic (BCC), or hexagonal close-packed (HCP) phases, rather than multiple complex phases [31].
- II. Solid Solution Phases: The high entropy effect favors the formation of solid solutions where different atoms are randomly distributed on the lattice sites. This random mixing can occur in FCC, BCC, or HCP structures, resulting in high configurational entropy that stabilizes these phases at high temperatures.[32, 33]
- III. Microstructure: The microstructure of HEAs can be highly varied and is influenced by the specific elemental composition and processing conditions. Typically, HEAs exhibit a single-phase or dual-phase microstructure with a mix of FCC and BCC phases. The presence of multiple principal elements can lead to lattice distortion, which plays a significant role in the mechanical properties of HEAs [34].
- 2.2. Exceptional Properties of HEAs
- The distinctive composition and structure of HEAs impart several unique properties that distinguish them from traditional alloys.
- I. Mechanical Strength: HEAs exhibit remarkable mechanical strength due to the combined effects of solid solution strengthening, lattice distortion, and the presence of multiple phases. These factors contribute to high yield strength and tensile strength [14].
- II. Wear and Corrosion Resistance: The random distribution of different atoms can lead to enhanced wear and corrosion resistance. The multiple elements can form a passive oxide layer that protects the alloy from aggressive environments [35, 36].
- III. High-Temperature Stability: HEAs maintain their structural integrity and mechanical properties at high temperatures. The high entropy stabilizes simple solid solution phases even at elevated temperatures, making them suitable for high-temperature applications.
- IV. Thermal Stability and Creep Resistance: HEAs exhibit excellent thermal stability and resistance to creep deformation, which is critical for applications in harsh thermal environments [37, 38].
- V. Magnetic and Electrical Properties: Depending on the constituent elements, HEAs can exhibit a range of magnetic and electrical properties, making them versatile for various applications, from structural components to functional materials [39].
- Thus, high-entropy alloys represent a paradigm shift in alloy design, offering a range of exceptional properties due to their unique compositional and structural characteristics. However, their development is hindered by several challenges that need to be addressed through continued research and innovation in materials science and engineering.
2. Fundamentals and background of High-Entropy Alloys
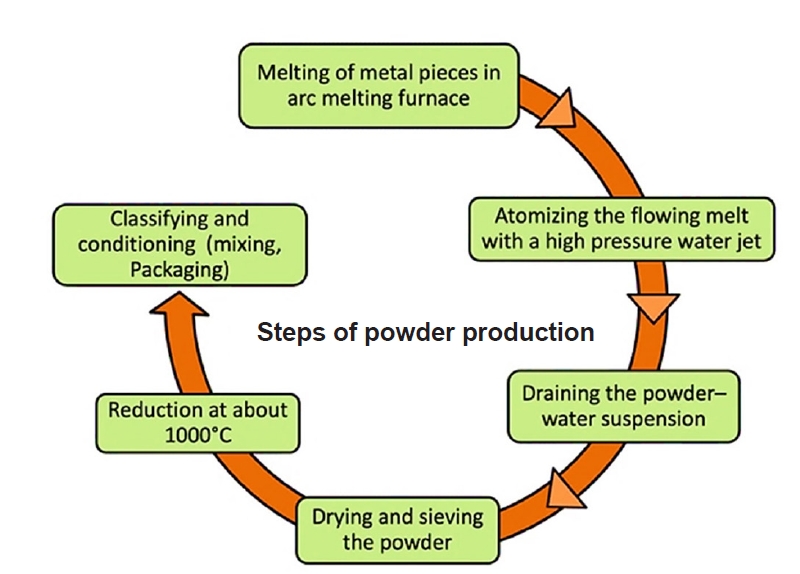
- Powder metallurgy (PM) is a manufacturing process that involves producing components from powdered materials through compaction and sintering. It offers a high degree of control over the final material properties and allows for the creation of complex shapes and unique material compositions. Fundamental steps are shown in Fig. 2.
- 3.1. Powder Production
- Atomization: Atomization is the most common method for producing metal powders, where molten metal is dispersed into fine droplets and solidified rapidly. Techniques include gas atomization, water atomization, and centrifugal atomization [40, 41].
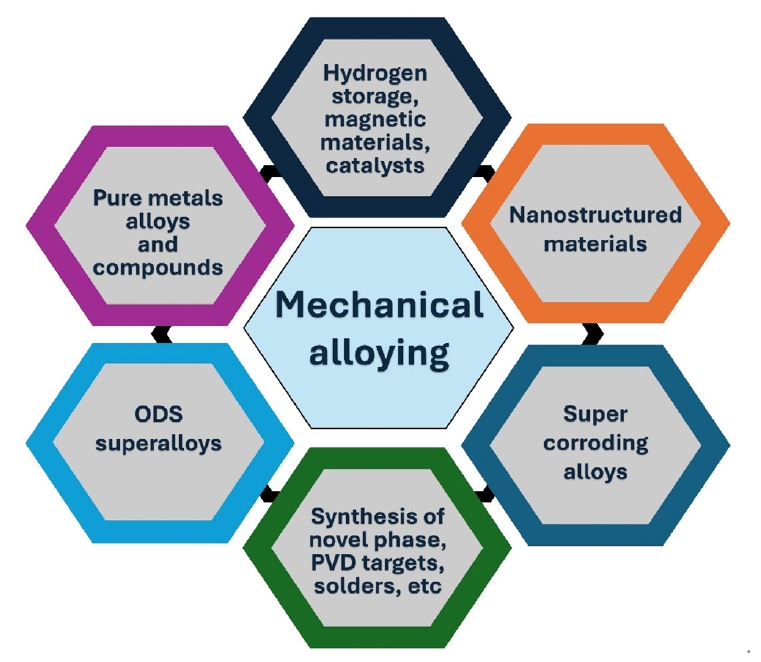
- Mechanical Alloying: This process involves repeated fracturing, welding, and re-welding of a blend of powders to create alloy powders. It is particularly useful for creating composite powders and nanostructured materials. A detailed illustration of the material synthesized through the mechanical alloying is presented in Fig. 3.
- Chemical Methods: These include reduction of metal oxides, electrolysis, and chemical vapor deposition (CVD). These methods can produce high-purity powders with controlled particle sizes.
- Atomization: Atomization is a primary method for producing metal powders in powder metallurgy (PM). It involves disintegrating molten metal into fine droplets, which then solidify into powder particles. This technique is crucial for creating powders with controlled particle sizes and shapes, essential for high-quality PM processes. The main atomization methods are gas atomization, water atomization, and plasma atomization, each with unique characteristics and applications. Different types of atomization methods are widely used for the production of alloy powder, some important methods are described in Table 1.
- 3.2. Sintering and Consolidation Methods in Powder Metallurgy
- Sintering and consolidation are critical processes in PM that transform compacted metal powders into solid, dense materials with enhanced mechanical properties. These processes involve heating the powder compact to promote bonding between particles, eliminate porosity, and achieve the desired microstructure and properties [44, 45]. Various sintering and consolidation methods are employed, each offering distinct advantages for different applications. Various types of processes have been used to consolidate the powder which are described in Table 2.
- 3.3. Advantages of PM in Alloy Development
- Powder metallurgy (PM) offers several significant advantages over traditional metallurgical processes, particularly in the development of advanced alloys such as HEAs. One of the most notable benefits is material efficiency. PM minimizes waste, as nearly all the powder can be utilized in the final product, making the process highly efficient in terms of material usage. This contrasts with conventional methods, which often involve substantial material loss due to machining and cutting [50].
- The ability to produce complex shapes and near-net shapes is another key advantage of PM. This capability reduces the need for extensive machining and post-processing, saving both time and resources. PM processes can create intricate geometries directly from the powder, allowing for greater design flexibility and the production of components that would be challenging or impossible to achieve with traditional methods. In addition, Uniform microstructure is a critical factor in determining the mechanical properties and performance of an alloy. PM excels in this area by producing materials with highly uniform microstructures. This uniformity enhances the mechanical properties, such as strength and durability, leading to superior performance in the final product. In addition, PM allows for precise control over the alloy composition, enabling the development of new alloys with tailored properties. This level of control is particularly beneficial for creating HEAs, which require specific elemental combinations to achieve their unique characteristics [11, 13, 51, 52]. In addition, chemical homogeneity is crucial for HEAs as it ensures uniform mechanical properties, corrosion resistance, and thermal stability by preventing localized weaknesses from elemental segregation. Powder metallurgy techniques like high-energy ball milling and homogeneous sintering are particularly effective in achieving this uniformity. High-energy ball milling breaks down segregated phases by repeatedly fracturing and cold-welding particles, promoting atomic-level mixing of elements. Homogeneous sintering then densifies the milled powders without melting, allowing controlled atomic diffusion for even elemental distribution. Together, these methods create a chemically homogeneous alloy microstructure, which significantly enhances HEA performance.
- Enhanced properties are another major advantage of PM. Processes such as mechanical alloying and SPS can significantly improve the mechanical properties of the material, including hardness, strength, and wear resistance. These enhancements are crucial for applications that demand high-performance materials capable of withstanding extreme conditions. Further, scalability is also a strength of PM. The process is suitable for both small-scale production of specialized components and large-scale industrial manufacturing, providing versatility in production volumes. This scalability makes PM an attractive option for various industries, from aerospace to biomedical, where both precision and volume production are required [11, 20].
- 3.4. Comparison with Other Processing Routes
- In comparison with conventional alloy production methods, PM stands out for its ability to efficiently utilize materials, produce complex shapes, ensure uniform microstructures, offer alloy design flexibility, enhance material properties, and scale production to meet diverse needs. These advantages make powder metallurgy a powerful and versatile tool in the development of advanced materials like high-entropy alloys, driving innovation and performance across multiple fields. Thus, a comparison has been made in Table 3.
- High-entropy alloys prepared by powder metallurgy exhibit outstanding mechanical and thermal properties, including high strength, hardness, toughness, wear resistance, and thermal stability. The PM process offers superior control over microstructure and composition compared to traditional casting and additive manufacturing. This results in materials with enhanced performance, particularly in high-temperature and demanding environments. As research and technology in powder metallurgy advance, the potential for HEAs in various high-performance applications continues to grow, highlighting the significance of this fabrication method in the development of advanced materials.
3. Powder Metallurgy Processes
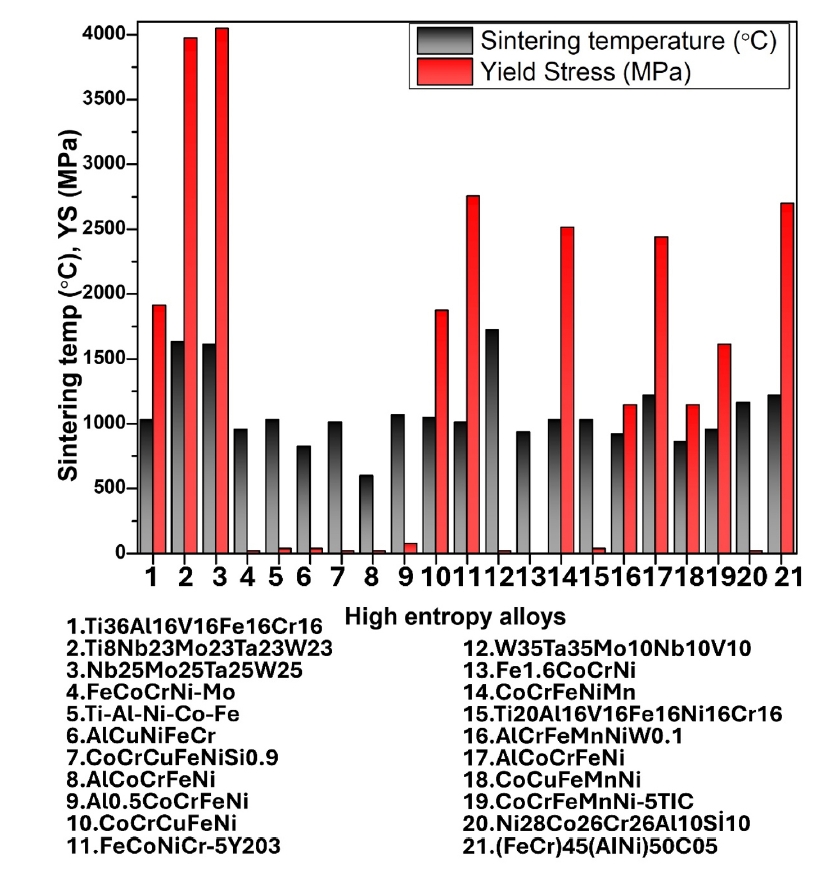
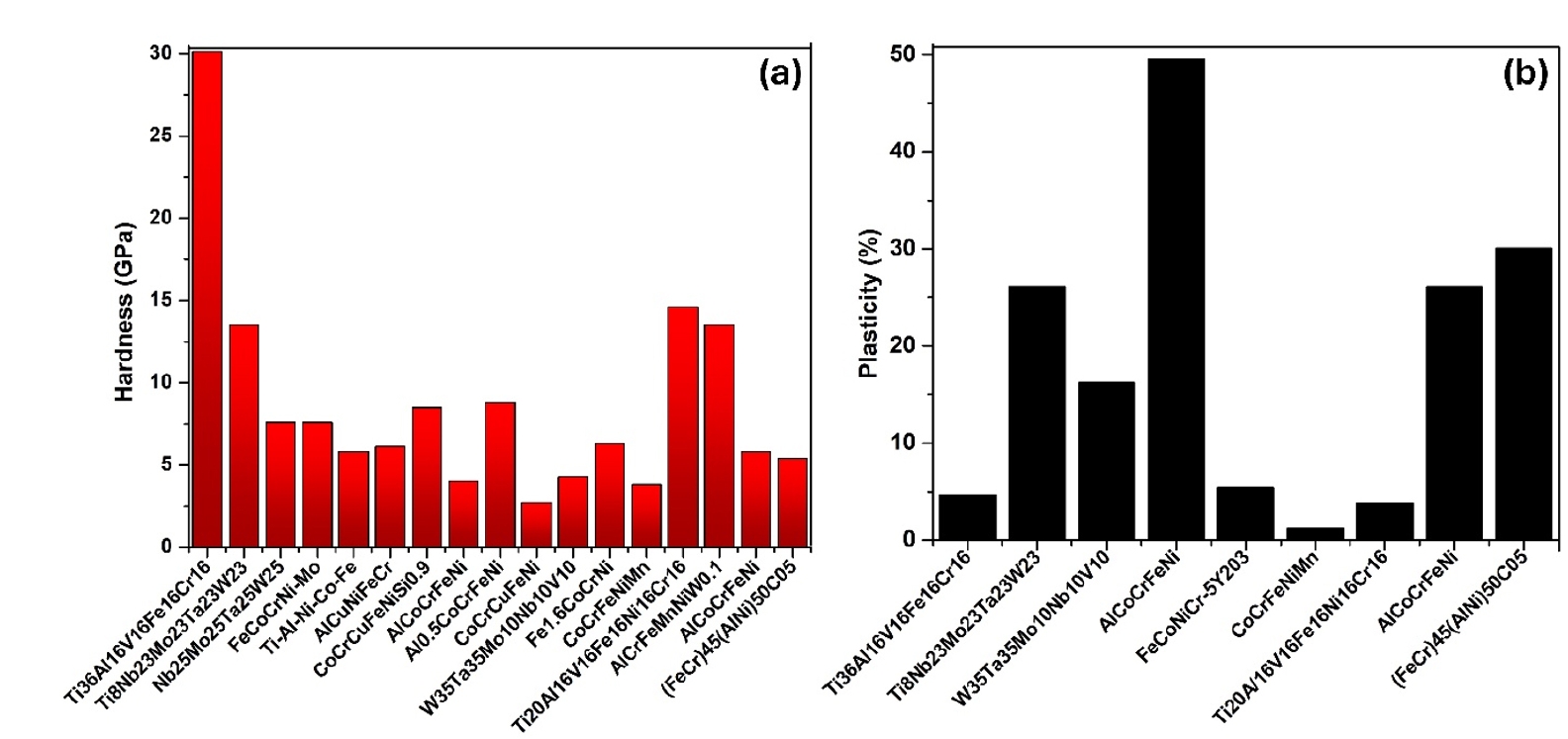
- HEAs synthesized by PM followed by milling and sintering exhibit remarkable mechanical properties due to their unique multi-component composition and refined microstructure. The PM process, particularly through high-energy ball milling, ensures a homogeneous distribution of elements at the atomic level, resulting in a fine and uniform microstructure. This leads to high strength and hardness as the multiple principal elements create lattice distortions that impede dislocation movement. Thus, in Fig. 4 and Fig. 5 (a & b), the mechanical properties of some reported HEAs have been compared and presented as a bar chart.
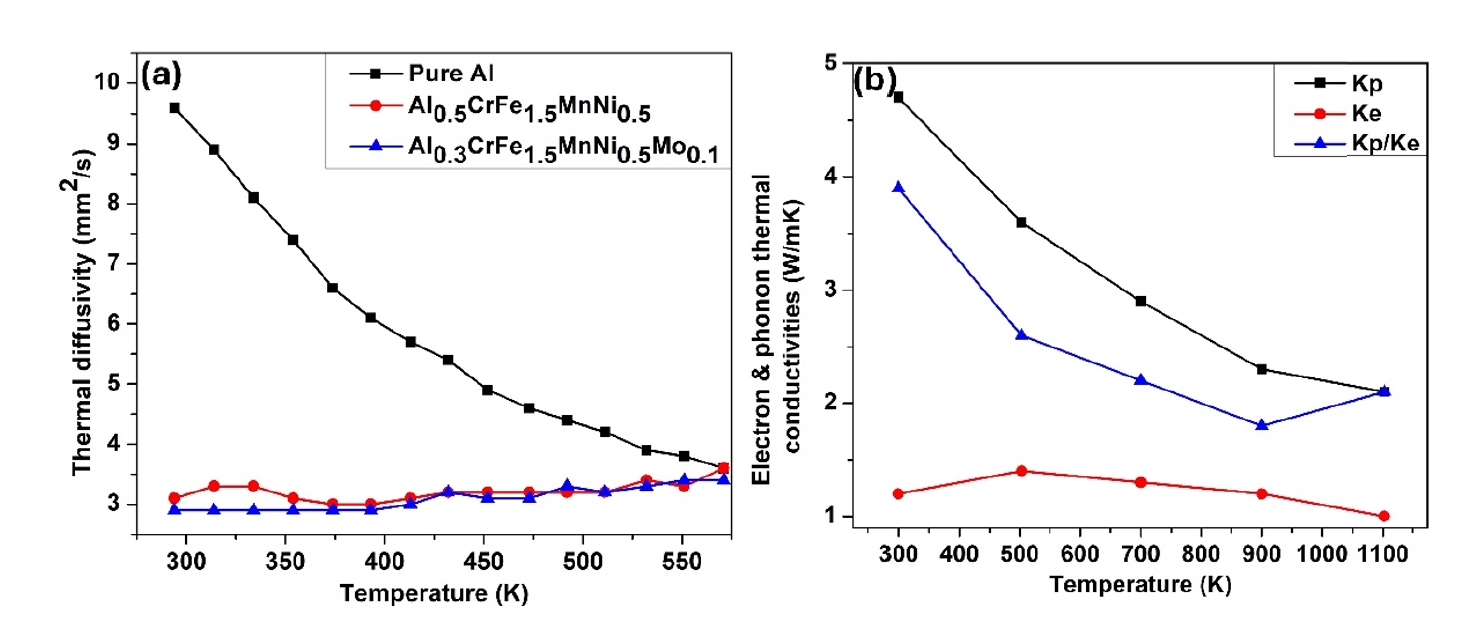
- Additionally, the enhanced toughness is attributed to the effective energy dissipation facilitated by the high-entropy effect, reducing the risk of brittle fracture. The superior hardness and toughness also translate into excellent wear resistance, making these HEAs suitable for abrasive environments. Furthermore, the uniform and fine-grained microstructure enhances fatigue resistance, enabling the alloys to endure cyclic loading conditions without premature failure. Overall, the mechanical properties of HEAs synthesized by PM followed by milling and sintering make them highly suitable for demanding applications that require a combination of high strength, toughness, and durability. In addition, Fig. 6 (a & b) presents the thermal properties of different high-entropy alloys.
- HEAs are distinguished by their unique multi-element composition, which imparts exceptional thermal properties. These alloys exhibit remarkable thermal stability, maintaining phase integrity and resisting phase transformations at elevated temperatures due to high configurational entropy. Their thermal conductivity is generally moderate to low, a result of phonon scattering by diverse atomic sizes and mass differences. This property, along with a reduced coefficient of thermal expansion, helps mitigate thermal stress and reduces thermal distortion. HEAs also demonstrate excellent thermal shock resistance, distributing thermal stress evenly and enhancing durability in environments with rapid temperature changes. Their high melting points extend their operational range in extreme thermal conditions, while superior oxidation resistance, facilitated by the formation of stable protective oxide layers, and good corrosion resistance at high temperatures ensure robust performance in harsh chemical environments. Furthermore, HEAs exhibit significant resistance to thermal fatigue, maintaining their properties through repeated heating and cooling cycles, which enhances the lifespan and reliability of components made from these materials. In addition, Table 4 and 5 shows the microstructure and their properties of different high entropy alloys.
4. Mechanical and Thermal Properties of HEAs
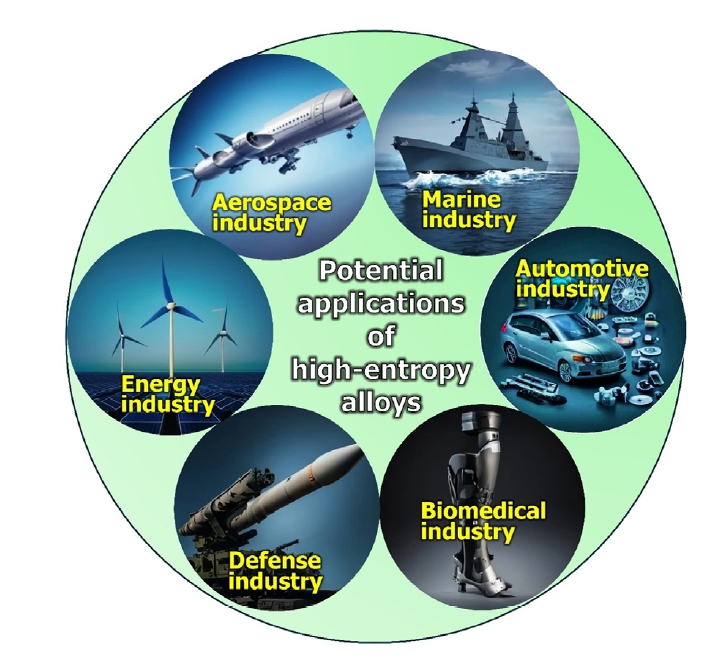
- HEAs produced by powder metallurgy have diverse and promising applications due to their unique properties, such as high thermal stability, mechanical strength, and corrosion resistance. The versatility and enhanced properties of HEAs produced by powder metallurgy make them suitable for a wide range of advanced engineering applications. A schematic presentation has been shown in Fig. 7.
- Aerospace industry: The aerospace industry has traditionally relied on superalloys and single-crystal alloys for components. However, the discovery of HEAs is shifting this narrative. HEAs offer high thermal stability and strength at elevated temperatures, making them ideal replacements for conventional alloys in jet engine components such as turbine blades, compressors, and combustors. For instance, GE Aviation has developed a NbMoTaW HEA to replace ferritic steel in rotors, offering superior properties like temperature resistance above 800°C, high strength, corrosion resistance, and sound creep resistance. Additionally, HEAs in other jet engine parts exhibit enhanced resistance to wear, corrosion, oxidation, fatigue, and creep compared to traditional superalloys and single-crystal alloys [55, 57].
- Automotive industry: HEAs are emerging as novel materials for the automotive industry due to their high strength and ductility. These properties make HEAs ideal for robust automotive components such as engine valves, gears, brake calipers, shafts, connecting rods, engine pistons, and ball joints. HEAs also offer resistance to fatigue, corrosion, wear, and impact loads, which are essential for automotive applications. For example, the AlCoCrFeNi HEA is lightweight, with a high yield strength of 1263 MPa at 773 K, ultimate compressive strength (UCS) of 1702 MPa, and plasticity of 19.9% up to 773 K. These characteristics make it suitable for structural and high-temperature applications like gears, engine pistons, and valves [58, 59].
- Marine industry: HEAs are becoming valuable in the nautical and maritime industry due to their high strength-to-weight ratio and excellent corrosion resistance. These properties are crucial for submarine machines and other maritime devices that are in constant contact with salt water, which is highly corrosive. Components for these applications require superior corrosion resistance and low density. One such HEA, MAR-M247 (NiCr16Co11Mo4), is a nickel-based alloy recognized for its effectiveness in constructing maritime ships and boats, offering enhanced durability and performance in harsh marine environments [60, 61].
- Biomedical industries: HEAs show great promise in biomedical applications, particularly for implants. Popescu et al. developed TiZrNbTaFe HEA, which exhibits superior corrosion resistance compared to the conventional Ti6Al4V alloy due to its single β phase and the presence of Ta, which forms a protective Ta2O5 film. This HEA has proven to be more biocompatible than Ti6Al4V, making it suitable for orthopedic implants like hip and knee replacements, as well as dental implants. CoCrFeMnNi HEA has shown superior performance in hip and knee replacements, while CoCrFeNiMnMo HEA has excelled in dental applications due to its corrosion resistance, biocompatibility, and strength. Additionally, HEAs are being explored as substitutes for conventional synthetic drugs [62–66].
- Energy industries: HEAs are emerging as superior materials for hydrogen energy storage, surpassing traditional metal hydrides like MgH2 and NaAlH4, which have a maximum hydrogen-to-metal (H/M) ratio of 2. Research indicates that HEAs can achieve an H/M ratio of 2.5 or more [67]. For instance, TiVZrNbHf HEA can store more hydrogen than its individual elements due to the large lattice strain in the alloy. This strain creates an environment conducive to absorbing hydrogen in both tetrahedral and octahedral interstitial sites, enhancing its storage capacity [68, 69].
- Defense Industries: HEAs are crucial in the military sector, particularly for military vehicles, protective shields, and armories. Their high strength, hardness, corrosion and wear resistance, impact strength, and fatigue resistance make them ideal for these applications. For instance, AerMet 100 (NiCoFeVMo) is used in ammunition casings due to its excellent wear and impact resistance. Research by Tang and Li using molecular dynamics simulations revealed that the ballistic performance of CrMnFeCoNi and CrFeCoNi HEAs is influenced by their dislocation dynamics under strain. CrFeCoNi HEA, with stronger atomic bonds and higher dislocation densities, exhibits superior strain-hardening and toughness, while CrMnFeCoNi, with weaker bonds and lower dislocation densities, is more susceptible to failure under ballistic impact. Thus, the presence of Mn reduces the impact energy of ballistic-resistant HEAs [70, 71].
5. Applications of HEAs Produced by Powder Metallurgy
- Despite their promising properties, the development and commercialization of HEAs face several significant challenges.
- (1) Complexity in Alloy Design: Designing HEAs involves selecting appropriate combinations of elements from a vast compositional space. The interactions between multiple elements are complex and not fully understood, making it challenging to predict the resulting phases and properties.
- (2) Manufacturing Difficulties: Traditional casting and processing methods may not be suitable for HEAs due to their compositional complexity. Achieving a homogeneous distribution of elements and preventing segregation during solidification are major challenges.
- (3) Characterization and Testing: The characterization of HEAs requires advanced techniques to accurately determine their phase composition, microstructure, and properties. High-throughput experimental methods and computational modeling are often necessary to understand the behavior of HEAs.
- (4) Cost and Scalability: The high cost of raw materials and the complexity of processing can make HEAs expensive to produce. Developing cost-effective and scalable manufacturing processes is crucial for their widespread adoption.
- (5) Limited Understanding of Properties: While HEAs exhibit unique properties, there is still limited understanding of how these properties change with different compositions and processing conditions. More research is needed to fully exploit the potential of HEAs.
- (6) In conclusion, the advancement of HEAs through powder metallurgy techniques marks a significant breakthrough in materials science. Powder metallurgy allows for precise control over composition and microstructure, leading to HEAs with superior mechanical properties, thermal stability, and corrosion resistance. This innovative approach not only enhances the performance and durability of HEAs but also broadens their application range across industries such as aerospace, automotive, medical, and energy. The synergy between HEA development and powder metallurgy techniques paves the way for the creation of next-generation materials that meet the stringent demands of modern engineering challenges. As research and technology continue to evolve, the potential of HEAs produced via powder metallurgy promises to revolutionize material design and application, driving forward industrial innovation and efficiency.
6. Conclusions and Challenges in HEA Development
-
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1A2C1005478).
-
Conflict of Interest
The authors declare no competing financial interests or personal relationships.
-
Data Availability Statement
All data generated or analyzed during this study are included in this published article
-
Author Information and Contribution
Sheetal Kumar Dewangan: Postdoctoral researcher, conceptualization, formal analysis, investigation, writing - original draft, writing - review & editing. Cheenepalli Nagarjuna: Postdoctoral researcher, formal analysis, investigation. Hansung Lee: Postdoctoral researcher, formal analysis, investigation. K. Raja Rao: Postdoctoral researcher, formal analysis, investigation. Man Mohan: Postdoctoral researcher, formal analysis, investigation. Reliance Jain: Professor, formal analysis, investigation. Byungmin Ahn: Professor, conceptualization, funding acquisition, supervision, writing - review & editing.
-
Acknowledgement
None.
Article information
| Methods | Process | Description | Advantages | Applications |
|---|---|---|---|---|
| Conventional sintering | Heating | Green compact is gradually heated to sintering temperature. | Simplicity | Structural components, |
| Soaking | Held at sintering temperature for a specific duration to enable diffusion and bonding. | Cost-effectiveness | Hard materials | |
| Cooling | Material is cooled at controlled rate to prevent thermal stresses. | |||
| Hot isostatic pressing (HIP) | Encapsulation | Powder compact is encapsulated in a gas-tight container. | High density | Aerospace components |
| Pressurization and heating | Encapsulated compact is subjected to high pressure and temperature in an autoclave using inert gas. | Isotropic properties | Biomedical implants | |
| Densification | Pressure and heat induce particle bonding and densification. | |||
| Spark plasma sintering (SPS) | Electric pulses | Pulsed electric current generates internal heat in the compact. | Fast processing | Nanomaterials |
| Pressure application | Uniaxial pressure applied simultaneously to promote densification. | Fine microstructures | Functionally graded materials | |
| Rapid sintering | Process is rapid, often completed in minutes. | |||
| Microwave sintering [46, 47] | Microwave energy | Microwaves generate heat, ensuring uniform temperature distribution. | Energy efficiency | Ceramics and composites |
| Sintering | Material is sintered via dielectric heating | Uniform heating | Low-temperature sintering | |
| Hot pressing [48, 49] | Compaction and heating | Powder is compacted and heated simultaneously in a die | High density and strength | Tool materials |
| Densification | Pressure and heat promote densification and grain growth. | Dimensional control | Refractory metals | |
| Cold isostatic pressing (CIP) | Encapsulation | Powder compact is placed in a flexible mold. | Uniform pressure | Preforms for sintering |
| Pressure application | Mold is placed in a pressure vessel; uniform pressure is applied using a liquid medium. | Shape flexibility | Large components | |
| Pre-sintering densification | Compact is densified before sintering process |
| Casting [53] | Powder metallurgy [11, 12] | Additive manufacturing [54] | |
|---|---|---|---|
| Microstructural control | Produces larger grain sizes and may lead to segregation of elements due to the slow cooling rates, resulting in inhomogeneous microstructures. | Achieves finer grain sizes and more uniform element distribution due to controlled powder production and sintering. | Can achieve complex geometries and near-net shapes with fine microstructures, but may suffer from anisotropy and residual stresses. |
| Produces uniform and isotropic properties with better control over porosity and grain size. | |||
| Mechanical properties | Typically yields materials with lower strength and hardness compared to powder metallurgy due to the presence of casting defects such as porosity and segregation. | Produces materials with higher strength, hardness, and improved toughness due to better microstructural control. | Offers high strength and hardness, but properties can vary depending on the build direction and process parameters. |
| Delivers consistent mechanical properties due to uniform microstructure and controlled processing conditions. | |||
| Thermal properties | Can result in lower thermal stability due to the potential for coarse intermetallic phases. | Provides higher thermal stability and oxidation resistance due to the homogeneous microstructure. | Exhibits good thermal properties, but residual stresses and anisotropy can affect performance at high temperatures. |
- 1. B. S. Murty, J. W. Yeh, S. Ranganathan and P. P. Bhattacharjee, In : B. S. Murty, J. W. Yeh, S. Ranganathan, P. P. Bhattacharjee, editors. High-Entropy Alloys, Second EdiElsevier, (2019) 13.
- 2. B. S. Murty, J. W. Yeh, S. Ranganathan and P. P. Bhattacharjee, In : B. S. Murty, J. W. Yeh, S. Ranganathan, editors. High-Entropy Alloys, Second EdiElsevier, Boston (2019) 1.
- 3. J. W. Yeh: JOM, 65 (2013) 1759.ArticlePDF
- 4. M. H. Tsai and J. W. Yeh: Mat. Res. Lett., 2 (2014) 107.Article
- 5. M. J. Chae, A. Sharma, M. C. Oh and B. Ahn: Met. Mater. Int., 27 (2021) 629.ArticlePDF
- 6. T. Zirari and V. Trabadelo: Heliyon, 10 (2024) e25867.Article
- 7. R. B. Nair, H. S. Arora, S. Mukherjee, S. Singh, H. Singh and H. S. Grewal: Ultrason. Sonochem., 41 (2018) 252.Article
- 8. L. Han, X. Xu, L. Wang, F. Pyczak, R. Zhou and Y. Liu: Mater. Res. Lett., 7 (2019) 3831.
- 9. M. Yue, H. Lambert, E. Pahon, R. Roche and S. Jemei: Renewable Sustainable Energy Rev., 146 (2021) 11118.
- 10. N. Kaushik, A. Meena and H. S. Mali: Mater. Manuf. Processes, 37 (2021) 1085.Article
- 11. G. S. Upadhyaya, Powder Metallurgy Technology, Cambridge International Science Pub, Cambridge (2002).
- 12. J. M. Torralba, Improvement of Mechanical and Physical Properties in Powder Metallurgy, Elsevier, (2014).
- 13. L. Moravcikova-Gouvea, I. Moravcik, V. Pouchly, Z. Kovacova, M. Kitzmantel, E. Neubauer and I. Dlouhy: Materials, 14 (2021) 5796.Article
- 14. L. Moravcikova-Gouvea, I. Moravcik, M. Omasta, J. Veselý, J. Cizek, P. Minárik, J. Cupera, A. Záděra, V. Jan and I. Dlouhy: Mater. Charact., 159 (2020) 110046.Article
- 15. V. Kruzhanov and V. Arnhold: Powder Metall., 55 (2012) 14.Article
- 16. T. DebRoy and J. W. Elmer: Mater. Today, 80 (2024) 737.Article
- 17. B. Ren, R. F. Zhao, A. Y. Jiang and Y. Yu: Micron, 158 (2022) 103291.Article
- 18. R. F. Zhao, B. Ren, B. Cai, Z. X. Liu, G. P. Zhang and J. J. Zhang: Results Phys., 15 (2019) 102667.Article
- 19. K. R. Rao, S. K. Dewangan, A. H. Seikh, S. K. Sinha and B. Ahn: Met. Mater. Int., 30 (2024) 726.ArticlePDF
- 20. S. H. Huo, M. Qian, G. B. Schaffer and E. Crossin: Fundamentals of Aluminium Metallurgy: Production, Processing and Applications, (2010) 655.
- 21. A. R. E. Singer: Powder Metall., 19 (1976) 4.Article
- 22. R. Jain, S. Jain, S. K. Dewangan, L. K. Boriwal and S. Samal: Journal of Alloys and Metallurgical Systems, 8 (2024) 100110.Article
- 23. S. K. Dewangan, S. Samal and V. Kumar: Mater. Today Commun., 27 (2021) 102356.Article
- 24. B. Cantor, I. T. H. Chang, P. Knight and A. J. B. Vincent: Mater. Sci. Eng., A, 375–377 (2004) 213.Article
- 25. K. H. Huang and J. W. Yeh, A Study on Multicomponent Alloy Systems Containing Equal-Mole Elements. National Tsing Hua University, M. S. thesis (1996).
- 26. M. H. Tsai and J. W. Yeh: Mater. Res. Lett., 2 (2014) 107.Article
- 27. S.K. Dewangan, A. Mangish, S. Kumar, A. Sharma, B. Ahn and V. Kumar: Eng. Sci. Technol. Int. J., 35 (2022) 101211.Article
- 28. J.-W. Yeh: Ann. Chim. Sci. Mat., 31 (2006) 633.Article
- 29. J. Li, Y. Huang, X. Meng and Y. Xie: Adv. Eng. Mater., 21 (2019) 1900343.Article
- 30. P.-K. Huang, J.-W. Yeh, T.-T. Shun and S.-K. Chen: Adv. Eng. Mater., 6 (2004) 74.Article
- 31. B. Chanda and J. Das: Adv. Eng. Mater., 20 (2017) 1700908.Article
- 32. X. Yang and Y. Zhang: Mater. Chem. Phys., 132 (2012) 233.Article
- 33. F. Tian: Front. Mater., 4 (2017) 1.Article
- 34. Y. Zhang, T. T. Zuo, Z. Tang, M. C. Gao, K. A. Dahmen, P. K. Liaw and Z. P. Lu: Prog. Mater. Sci., 61 (2014) 1.Article
- 35. Y. Ren, Q. Zhou, D. Hua, Z. Huang, Y. Li, Q. Jia, P. Gumbsch, C. Greiner, H. Wang and W. Liu: Sci. Bull., 69 (2024) 227.Article
- 36. D. Banik, S. Mukherjee, H. Fujiwara, K. Ameyama and K. Mondal: Wear, 534–535 (2023) 205125.Article
- 37. A. Karati, K. Guruvidyathri, V. S. Hariharan and B. S. Murty: Scr. Mater., 162 (2019) 465.Article
- 38. Z. Xu, H. Zhang, W. Li, A. Mao, L. Wang, G. Song and Y. He: Addit. Manuf., 28 (2019) 766.Article
- 39. Y. F. Kao, S. K. Chen, T. J. Chen, P. C. Chu, J. W. Yeh and S. J. Lin: J. Alloys Compd., 509 (2011) 1607.Article
- 40. D. J. Hodkin, J. S. Pollock and P. W. Sutcliffe: Powder Metall., 19 (1976) 12.Article
- 41. D. Yim, P. Sathiyamoorthi, S. J. Hong and H. S. Kim: J. Alloys Compd., 781 (2019) 389.Article
- 42. B. S. Murty, J. W. Yeh, S. Ranganathan, P. P. Bhattacharjee: B. S. Murty, J. W. Yeh, S. Ranganathan, P. P. Bhattacharjee (Eds.), High-Entropy Alloys (Second Edition), Second Edi, Elsevier, (2019) 103.
- 43. A. Kumar, S. Kumar Dewangan, S. Singh, M. Chopkar and R. B. Sreesha: High-Entropy Alloys, (2023) 31.
- 44. S. K. Dewangan: Indian Institute of Technology Indore, (2021).
- 45. S. K. Dewangan, D. Kumar, S. Samal and V. Kumar: J. Mater. Eng. Perform., 30 (2021) 4421.ArticlePDF
- 46. P. Veronesi, R. Rosa, E. Colombini and C. Leonelli: Technologies, Basel, 3 (2015) 182.Article
- 47. W. Wong and M. Gupta: Technologies Basel, 3 (2015) 1.Article
- 48. C. Nagarjuna, S.K. Dewangan, H. Lee and B. Ahn: Mater. Sci. Eng., A, 886 (2023) 145680.Article
- 49. X. Liu, H. Cheng, Z. Li, H. Wang, F. Chang, W. Wang, Q. Tang and P. Dai: Vacuum, 165 (2019) 297.Article
- 50. S. K. Dewangan, C. Nagarjuna, H. Lee, A. Sharma and B. Ahn: Powder Metall., 66 (2023) 650.ArticlePDF
- 51. A. N. Klein, R. P. Cardoso, H. C. Pavanati, C. Binder, A. M. Maliska, G. Hammes, D. Fusao, A. Seeber, S. F. Brunatto and J. L. R. Muzart: Plasma Sci. Technol., 15 (2013) 70.Article
- 52. E. Colombini, R. Rosa, L. Trombi, M. Zadra, A. Casagrande and P. Veronesi: Mater. Chem. Phys., 210 (2018) 78.Article
- 53. M. E. Glicksman, Principals of Solidification An Introduction to Modern Casting and Crystal Growth Concepts, Springer, (2011).
- 54. S. Chen, Y. Tong and P. Liaw: Entropy, 20 (2018) 937.Article
- 55. C. O. Ujah, D. V. V. Kallon and V. S. Aigbodion: Mater. Today Sustainability, 25 (2024) 100639.Article
- 56. B. Zhang, Y. Huang, Z. Dou, J. Wang and Z. Huang: Adv. Mater. Devices, 9 (2024) 100688.Article
- 57. D.O. Svensson: High Entropy Alloys: Breakthrough Materials for Aero Engine Applications, (2015).
- 58. M. G. Perspectives and G. Mazzolai: Recent Patents on Materials Science, 5 (2012) 137.Article
- 59. Q. Tian, G. Zhang, K. Yin, W. Wang, W. Cheng and Y. Wang: Mater. Charact., 151 (2019) 302.Article
- 60. M. N. Rao: Trans. Indian Inst. Met., 61 (2008) 87.ArticlePDF
- 61. X. Gong, Y. Yu, T. Wang, Y. Liu, L. Zhang, Z. Gao, H. Ziyong, X. Chen, S. He and X. Qu: Met. Mater. Int., 29 (2023) 3286.ArticlePDF
- 62. Y. Guo, X. Li and Q. Liu: Mater. Des., 196 (2020) 109085.Article
- 63. O. Bazaka, K. Bazaka, P. Kingshott, R. J. Crawford and E. P. Ivanova: The Chemistry of Inorganic Biomaterials, (2021) 1.
- 64. W. Y. Ching, S. San, J. Brechtl, R. Sakidja, M. Zhang and P. K. Liaw: NPJ Comput. Mater., 6 (2020) 45.Article
- 65. G. Shibo, Q. Xuanhui, H. Xinbo, Z. Ting and D. Bohua: J. Mater. Process Technol., 173 (2006) 310.Article
- 66. A. T. Sidambe: Materials, 7 (2014) 8168.Article
- 67. M. Sahlberg, D. Karlsson, C. Zlotea and U. Jansson: Sci Rep, 6 (2016) 36770.Article
- 68. M. Fu, X. Ma, K. Zhao, X. Li and D. Su: iScience, 24 (2021) 102177.Article
- 69. Office-of-Energy-Efficiency-&-Renewable-Energy, Materials-Based Hydrogen Storage | Department of Energy, (2015).
- 70. S. Siengchin: Def. Techno., 24 (2023) 1.Article
- 71. J. L. Dong, F. C. Li, Z. P. Gu, M. Q. Jiang, Y. H. Liu, G. J. Wang and X. Q. Wu: Int. J. Plast., 171 (2023) 103801.Article
References
Figure & Data
References
Citations

- Fabrication and Alloying Behavior of Ultra-Lightweight AlTiCrVMg High-Entropy Alloy via Al-Mg Mutual Solubility and Sintering Control
Eunhyo Song, Hansung Lee, Byungmin Ahn
Journal of Powder Materials.2025; 32(3): 254. CrossRef - Thermodynamic and Electronic Descriptor-Driven Machine Learning for Phase Prediction in High-Entropy Alloys: Experimental Validation
Nguyen Lam Khoa, Nguyen Duy Khanh, Hoang Thi Ngoc Quyen, Nguyen Thi Hoang, Oanh, Le Hong Thang, Nguyen Hoa Khiem, Nguyen Hoang Viet
Journal of Powder Materials.2025; 32(3): 191. CrossRef
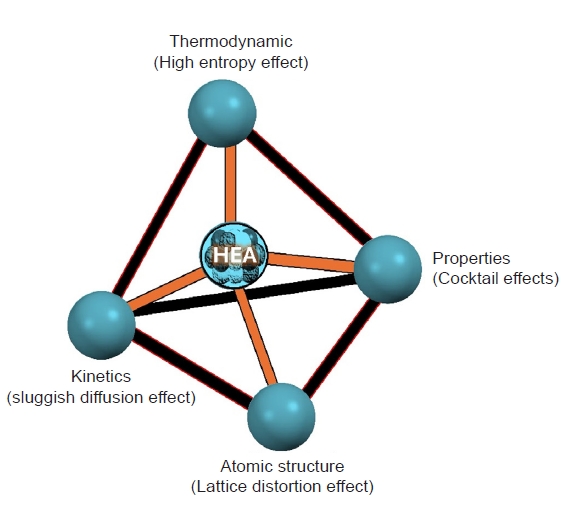
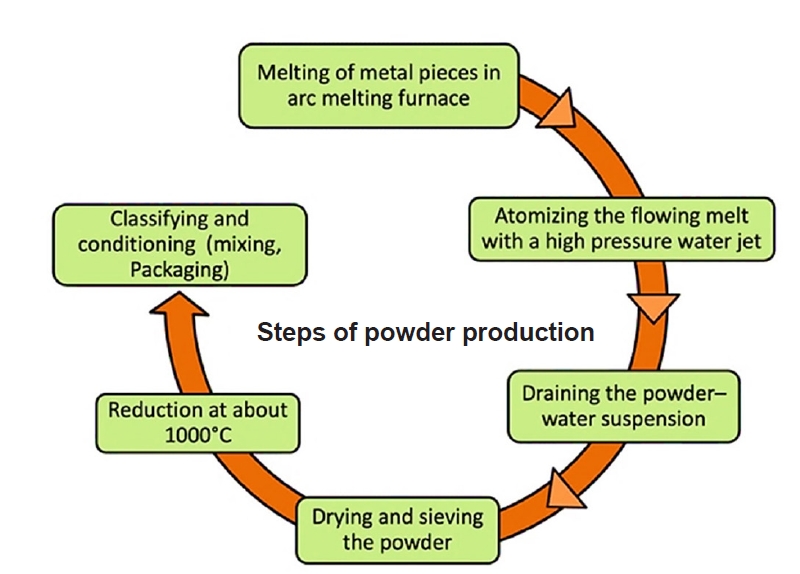
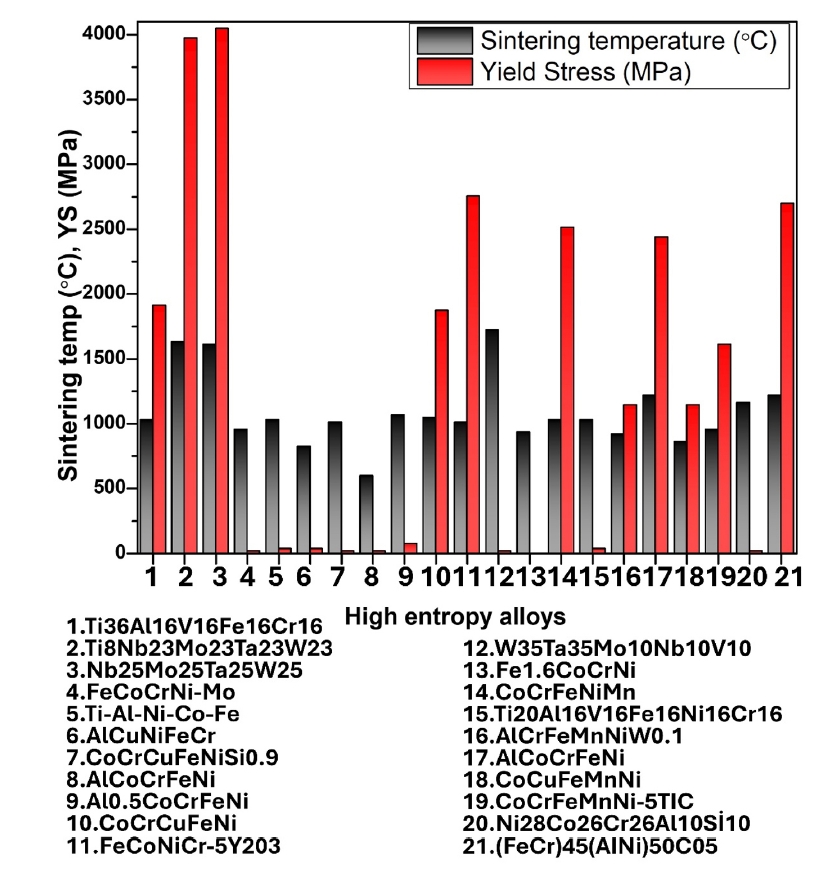
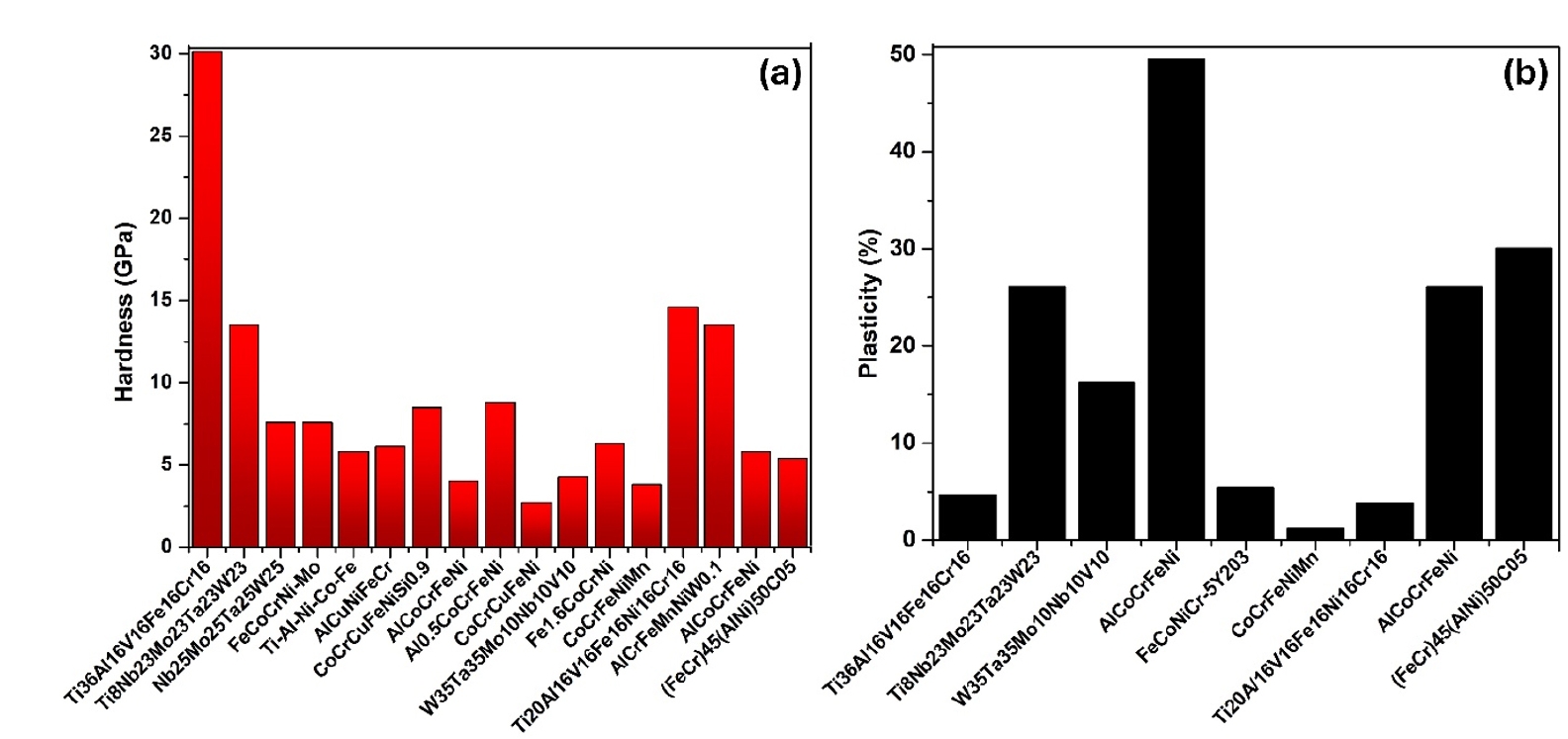
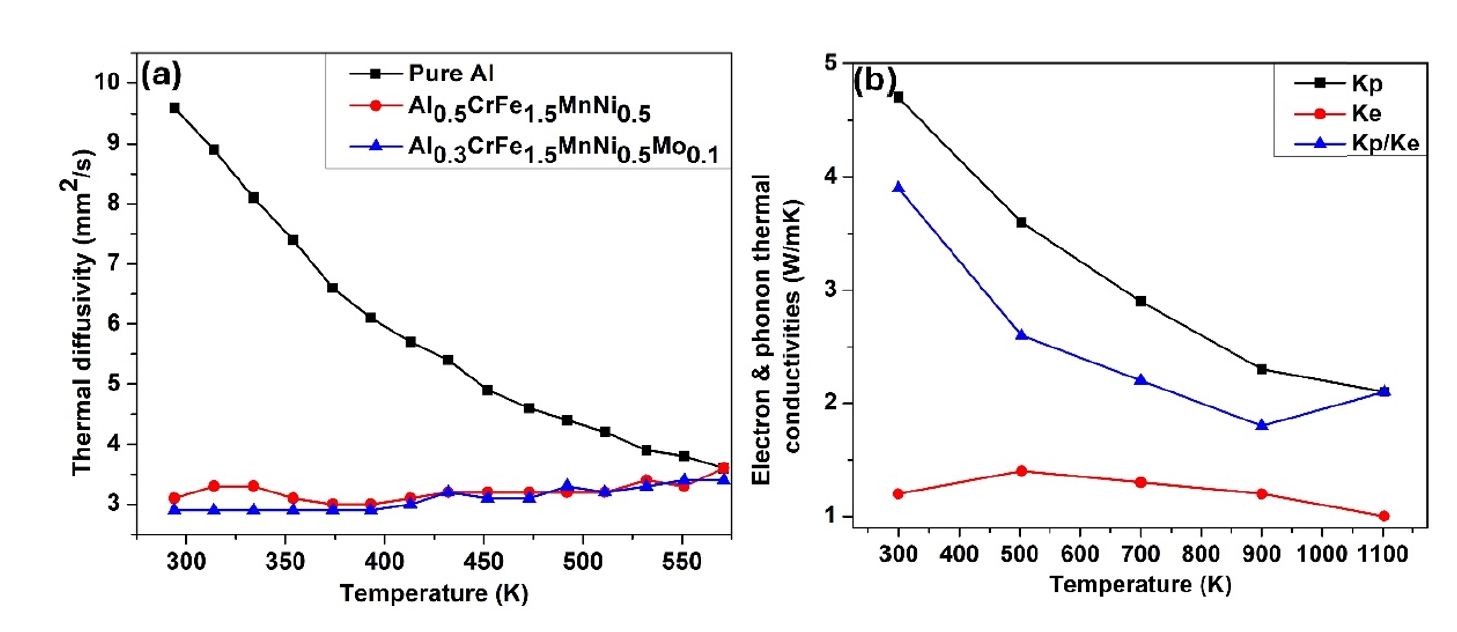
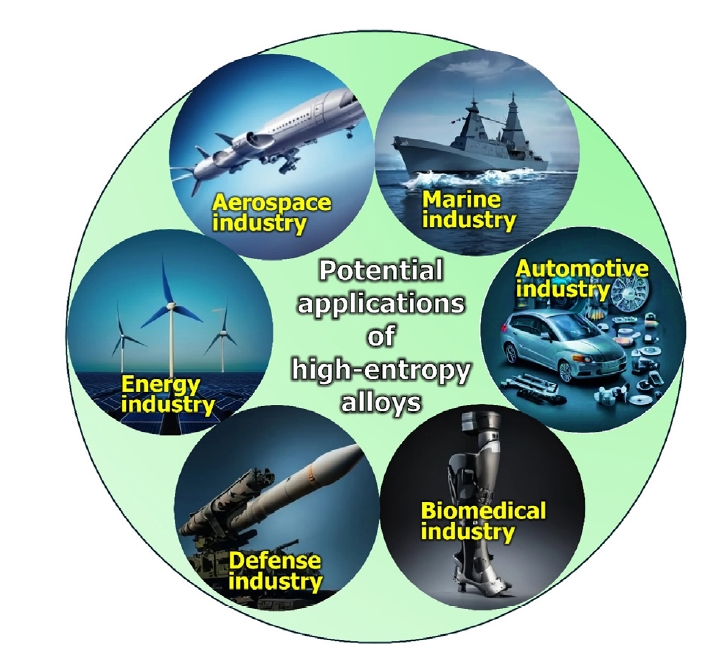
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
Fig. 7.
| Methods | Process | Description | Advantages | Applications |
|---|---|---|---|---|
| Gas atomization | Molten metal stream | Melted in crucible and poured through nozzle | Spherical particles Controlled particle size High purity | Aerospace and automotive Additive manufacturing |
| Gas stream | High-pressure gas jets break molten stream into droplets | |||
| Cooling and solidification | Droplets rapidly solidify in atomization chamber, forming fine powder. | |||
| Water atomization | Molten metal Stream | Metal is melted and poured through a tundish into water-cooled chamber. | Cost-effective High production rates Diverse particle shapes | Structural components Hard materials |
| Water jets | High-pressure water jets break molten stream into droplets. | |||
| Cooling and solidification | Droplets solidify quickly due to high cooling rate from water. | |||
| Plasma atomization | Plasma torch | Plasma torch melts metal feedstock (wire or rod) at high temperatures. | High-purity spherical particles Fine powders | Aerospace and biomedical Additive manufacturing |
| Inert gas stream | Inert gas (argon) atomizes molten metal into fine droplets. | |||
| Cooling and solidification | Droplets solidify in atomization chamber, forming fine powder particles. |
| Methods | Process | Description | Advantages | Applications |
|---|---|---|---|---|
| Conventional sintering | Heating | Green compact is gradually heated to sintering temperature. | Simplicity | Structural components, |
| Soaking | Held at sintering temperature for a specific duration to enable diffusion and bonding. | Cost-effectiveness | Hard materials | |
| Cooling | Material is cooled at controlled rate to prevent thermal stresses. | |||
| Hot isostatic pressing (HIP) | Encapsulation | Powder compact is encapsulated in a gas-tight container. | High density | Aerospace components |
| Pressurization and heating | Encapsulated compact is subjected to high pressure and temperature in an autoclave using inert gas. | Isotropic properties | Biomedical implants | |
| Densification | Pressure and heat induce particle bonding and densification. | |||
| Spark plasma sintering (SPS) | Electric pulses | Pulsed electric current generates internal heat in the compact. | Fast processing | Nanomaterials |
| Pressure application | Uniaxial pressure applied simultaneously to promote densification. | Fine microstructures | Functionally graded materials | |
| Rapid sintering | Process is rapid, often completed in minutes. | |||
| Microwave sintering [46, 47] | Microwave energy | Microwaves generate heat, ensuring uniform temperature distribution. | Energy efficiency | Ceramics and composites |
| Sintering | Material is sintered via dielectric heating | Uniform heating | Low-temperature sintering | |
| Hot pressing [48, 49] | Compaction and heating | Powder is compacted and heated simultaneously in a die | High density and strength | Tool materials |
| Densification | Pressure and heat promote densification and grain growth. | Dimensional control | Refractory metals | |
| Cold isostatic pressing (CIP) | Encapsulation | Powder compact is placed in a flexible mold. | Uniform pressure | Preforms for sintering |
| Pressure application | Mold is placed in a pressure vessel; uniform pressure is applied using a liquid medium. | Shape flexibility | Large components | |
| Pre-sintering densification | Compact is densified before sintering process |
| Casting [53] | Powder metallurgy [11, 12] | Additive manufacturing [54] | |
|---|---|---|---|
| Microstructural control | Produces larger grain sizes and may lead to segregation of elements due to the slow cooling rates, resulting in inhomogeneous microstructures. | Achieves finer grain sizes and more uniform element distribution due to controlled powder production and sintering. | Can achieve complex geometries and near-net shapes with fine microstructures, but may suffer from anisotropy and residual stresses. |
| Produces uniform and isotropic properties with better control over porosity and grain size. | |||
| Mechanical properties | Typically yields materials with lower strength and hardness compared to powder metallurgy due to the presence of casting defects such as porosity and segregation. | Produces materials with higher strength, hardness, and improved toughness due to better microstructural control. | Offers high strength and hardness, but properties can vary depending on the build direction and process parameters. |
| Delivers consistent mechanical properties due to uniform microstructure and controlled processing conditions. | |||
| Thermal properties | Can result in lower thermal stability due to the potential for coarse intermetallic phases. | Provides higher thermal stability and oxidation resistance due to the homogeneous microstructure. | Exhibits good thermal properties, but residual stresses and anisotropy can affect performance at high temperatures. |
| HEA | Observed phase(s) through different processing route(s) | Effects on mechanical properties | ||||
|---|---|---|---|---|---|---|
| Composition | Melting and casting | MA SPS | AM | Melting and casting | MA SPS | AM |
| CoCrFeNiMn | FCC | FCC | FCC + BCC | Compressive strength: 1987 MPa | Tensile strength: 601 MPa | |
| Hardness: 646 HV | ||||||
| CoCrFeNiAl03 | FCC | FCC + BCC | FCC | UTS: 528 MPa | Compressive strength: 1907 MPa | YS: 730 MPa |
| YTS: 275 MPa | Hardness: 625 HV | UTS: 896 MPa | ||||
| CoCrFeNi | FCC + Cr7C3 | FCC | Hardness: 580 HV | |||
| AICoCrCuFeNi | FCC + BCC | FCC + BCC | BCC | Hardness: 515.5 HV (5.056 GPa) Compressive strength: 1.82 GPa | Hardness: 8.13 GPa | |
| Elastic modulus: 172 GPa | ||||||
| TiZrNbMon3 V0.3 | BCC | FCC BCC | Yield strength: 1312 MPa and 50% increase in plastic strain | |||
| Ni155015CrFeT105 | FCC | FCC | YS: 896 MPa Compressive strength:1502 MPa Hardness: 515 HV | Hardness: 442 HV 0.3 | ||
| Tensile strength: 1384 MPa | ||||||
| Elastic modulus:216 GPa | ||||||
| Alloys | Processing | Density (g/cm3) | Phases | Yield strength |
|---|---|---|---|---|
| Ti0.5VNbMoTa | MA+SPS | 9.99 | BCC | 2563 |
| Ti1VNbMoTa | MA+SPS | 9.45 | BCC | 2208 |
| Ti1.5VNbMoTa | MA+SPS | 9.08 | BCC+FCC | 2696 |
| Ti2VNbMoTa | MA+SPS | 8.75 | BCC+FCC | 2824 |
| NbMoTaWVCr | MA+SPS | 11.06 | BCC+Laves+Oxide | 3410 |
| NbMoTaWVCr | MA+SPS | 11.16 | BCC+Laves+Oxide | 3416 |
| MoNbTaTiV | MA+SPS | 9.45 | BCC | 2208 |
| NbMoTaWVTi | MA+SPS | 10.6 | BCC+TiO | 2709 |
| CrNbVMo | MA+SPS | 8.03 | BCC+NbO0.7 | 2743 |
| Al0.5CrNbVMo | MA+SPS | 7.53 | BCC+Al2O3 | 2497 |
| Al1CrNbVMo | MA+SPS | 7.05 | BCC+Al2O3 | 2326 |
| (W35Ta35Mo15Nb15)95Ni5 | MA+SPS | 14.55 | BCC+Nb5.7Ni4Ta2.3O2 | 2128 |
| NbTaWMo | MA+SPS | 13.44 | BCC+Silicide | 1217 |
| NbTaWMoSi0.25 | MA+SPS | 12.92 | BCC+Silicide | 1826 |
| NbTaWMoSi0.5 | MA+SPS | 12.65 | BCC+Silicide | 1883 |
| NbTaWMoSi0.75 | MA+SPS | 12.23 | BCC+Silicide | 2483 |
| Al0.1CrMoNbV | MA+SPS | 7.97 | BCC+Al2O3 | 2544 |
| Al0.1CrMoNbVB0.015 | MA+SPS | 7.97 | BCC+Al2O3 | 2933 |
| TiNbTa0.5Zr | Sintering | 7.6 | BCC | 1310 |
| TiNbTa0.5ZrAl0.2 | Sintering | – | BCC | 1500 |
| TiNbTa0.5ZrAl0.5 | Sintering | 7.3 | BCC | 1740 |
| W0.3(TaTiCrV)0.7 | SPS | 13.4 | BCC | 2265 |
| W0.4(TaTiCrV)0.6 | SPS | 13.6 | BCC | 2314 |
| W0.5(TaTiCrV)0.5 | SPS | 14.5 | BCC | 2144 |
| W0.6(TaTiCrV)0.4 | SPS | 14.9 | BCC | 2187 |
| W0.7(TaTiCrV)0.3 | SPS | 15.7 | BCC | 1473 |
| W0.8(TaTiCrV)0.2 | SPS | 16.5 | BCC | 1208 |
| W0.9(TaTiCrV)0.1 | SPS | 16.5 | BCC | 1206 |
| V0.5Nb0.5ZrTi | SLM | 6.5 | BCC | 1450 |
Table 1.
Table 2.
Table 3.
Table 4.
Table 5.
TOP
 kpmi
kpmi

 ePub Link
ePub Link Cite this Article
Cite this Article







