- [English]
- A Study on the Removal of Heavy Metal with Mg-Modified Zeolite
-
Jei-Pil Wang, Gyu-Cheol Kim, Min-Seok Go
-
J Korean Powder Metall Inst. 2020;27(4):287-292. Published online August 1, 2020
-
DOI: https://doi.org/10.4150/KPMI.2020.27.4.287
-
-
1,845
View
-
23
Download
-
2
Citations
-
 Abstract Abstract
 PDF PDF
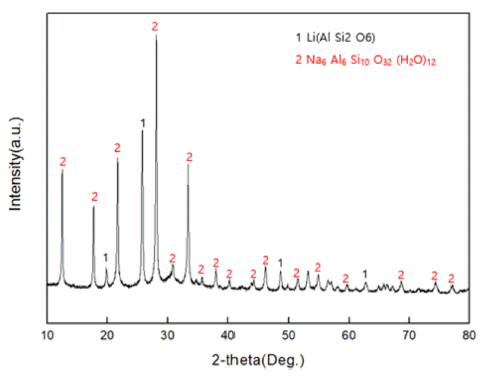
The subject of this study is a zeolite generated as a by-product of recycling LAS (lithium-aluminum-silicate) resources, a kind of glass and ceramic produced by induction. The zeolite by-product is modified into Mg-zeolite using Mg as a cation to absorb Pb, a heavy metal generated from water pollution caused by recent industrial wastewater. An ion-exchange method is used to carry out the modification process, from zeolite byproduct to Mg-zeolite, and simultaneously absorb the Pb in the heavy-metal solution (99.032 mg/L). It is found that the sodium zeolite in the raw material residue can be modified to magnesium zeolite by reacting it with a mixture solution at 1 M concentration for 24 h. As a result, it is found that the residual Pb (0.130 mg/L) in the heavy metal solution is shown to be absorbed by 99.86%, with successful formation of a Mg-modified zeolite. -
Citations
Citations to this article as recorded by  - Penentuan Kualitas Kaolin sebagai Prekursor Sintesis Zeolit pada Kegiatan Praktikum
Rohmat Ismail, Manasye Erlangga, Ari Himawan, Givana Indah Nurul Afiah
Jurnal Pengelolaan Laboratorium Pendidikan.2025; 7(2): 81. CrossRef - Y-Type Zeolite Synthesized from an Illite Applied for Removal of Pb(II) and Cu(II) Ions from Aqueous Solution: Box-Behnken Design and Kinetics
Kinjal J. Shah, Jiacheng Yu, Ting Zhang, Zhaoyang You
Water.2023; 15(6): 1171. CrossRef
- [English]
- Synthesis of Nanosized Nickel Particle from Spent Cathodic Material Containing Lithium
-
Jei-Pil Wang
-
J Korean Powder Metall Inst. 2019;26(4):340-344. Published online August 1, 2019
-
DOI: https://doi.org/10.4150/KPMI.2019.26.4.340
-
-
 Abstract Abstract
 PDF PDF
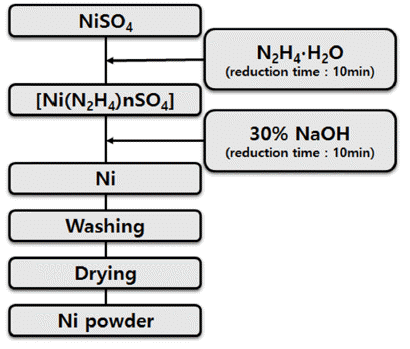
Due to the rapid development of electricity, electronics, information communication, and biotechnology in recent years, studies are actively being conducted on nanopowders as it is required not only for high strengthening but also for high-function powder with electric, magnetic, and optical properties. Nonetheless, studies on nickel nanopowders are rare. In this study of the synthesis of nickel nanoparticles from LiNiO2 (LNO), which is a cathode active material, we have synthesized the nanosized nickel powder by the liquid reduction process of NiSO4 obtained through the leaching and purification of LNO. Moreover, we have studied the reduction reaction rate according to the temperature change of liquid phase reduction and the change of particle size as a function of NaOH addition amount using hydrazine monohydrate (N2H4·H2O) and NaOH.
- [English]
- A Study on the Recovery of Li2CO3 from Cathode Active Material NCM(LiNiCoMnO2) of Spent Lithium Ion Batteries
-
Jei-Pil Wang, Jae-Jung Pyo, Se-Ho Ahn, Dong-Hyeon Choi, Byeong-Woo Lee, Dong-Won Lee
-
J Korean Powder Metall Inst. 2018;25(4):296-301. Published online August 1, 2018
-
DOI: https://doi.org/10.4150/KPMI.2018.25.4.296
-
-
3,496
View
-
76
Download
-
11
Citations
-
 Abstract Abstract
 PDF PDF
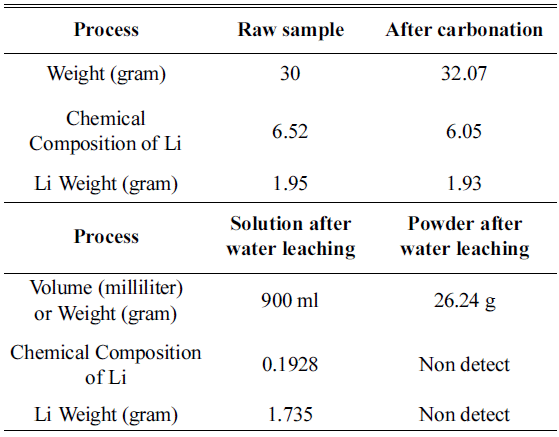
In this study, an experiment is performed to recover the Li in Li2CO3 phase from the cathode active material NMC (LiNiCoMnO2) in waste lithium ion batteries. Firstly, carbonation is performed to convert the LiNiO, LiCoO, and Li2MnO3 phases within the powder to Li2CO3 and NiO, CoO, and MnO. The carbonation for phase separation proceeds at a temperature range of 600°C~800°C in a CO2 gas (300 cc/min) atmosphere. At 600~700°C, Li2CO3 and NiO, CoO, and MnO are not completely separated, while Li and other metallic compounds remain. At 800 °C, we can confirm that LiNiO, LiCoO, and Li2MnO3 phases are separated into Li2CO3 and NiO, CoO, and MnO phases. After completing the phase separation, by using the solubility difference of Li2CO3 and NiO, CoO, and MnO, we set the ratio of solution (distilled water) to powder after carbonation as 30:1. Subsequently, water leaching is carried out. Then, the Li2CO3 within the solution melts and concentrates, while NiO, MnO, and CoO phases remain after filtering. Thus, Li2CO3 can be recovered. -
Citations
Citations to this article as recorded by  - Selective lithium Pre-Leaching from spent NMC black mass via pyrolysis (Carbothermal thermal Treatment) and controlled leaching
Amir Hossein Mohammad Zadeh, Spencer Cunnigham, Devon Gray, Sevan Bedrossian, Baian Almusned, Jeffrey Daniel Henderson, Gisele Azimi
Waste Management.2026; 212: 115339. CrossRef - Hydrogen Reduction of Black Mass Recovered from NCM-based Spent Lithium-ion Batteries
Jae-Ho Hwang, Sang-Yeop Lee, So-Yeong Lee, Ho-Sang Sohn
Resources Recycling.2025; 34(3): 34. CrossRef - Reduction Roasting of Cathode Materials of NCM Based Lithium-ion Batteries Using CH4(g)
Jae-Ho Hwang, Sang-Yeop Lee, Ho-Sang Sohn
Resources Recycling.2025; 34(4): 48. CrossRef - High‐Rate Rechargeable Li/SOCl2 Batteries Enabled by Cobalt Phthalocyanine Cathodic Catalysts
Yingxuan Song, Haibo Ouyang, Zhanwei Xu, Zeyang Zhang, Kang Li, Jianfeng Huang, Zhi Li, Tian Wang, Jun Yang
Small.2025;[Epub] CrossRef - Reduction Roasting of Black Mass Recovered from NCM-based Spent Lithium-ion Batteries Using CH4 Gas
Sang-Yeop Lee, Jae-Ho Hwang, Ho-Sang Sohn
Resources Recycling.2025; 34(5): 93. CrossRef - Metals Recovery from Spent Lithium-ion Batteries Cathode Via Hydrogen Reduction-water Leaching-carbothermic or Hydrogen Reduction Process
Tahereh Rostami, Behnam Khoshandam
Mining, Metallurgy & Exploration.2024; 41(3): 1485. CrossRef - Influence of Flow-Gas Composition on Reaction Products of Thermally Treated NMC Battery Black Mass
Christin Stallmeister, Bernd Friedrich
Metals.2023; 13(5): 923. CrossRef - Holistic Investigation of the Inert Thermal Treatment of Industrially Shredded NMC 622 Lithium-Ion Batteries and Its Influence on Selective Lithium Recovery by Water Leaching
Christin Stallmeister, Bernd Friedrich
Metals.2023; 13(12): 2000. CrossRef - Environmentally Friendly Recovery of Lithium from Lithium–Sulfur Batteries
Lilian Schwich, Bernd Friedrich
Metals.2022; 12(7): 1108. CrossRef - Early-Stage Recovery of Lithium from Tailored Thermal Conditioned Black Mass Part I: Mobilizing Lithium via Supercritical CO2-Carbonation
Lilian Schwich, Tom Schubert, Bernd Friedrich
Metals.2021; 11(2): 177. CrossRef - Exploring a green route for recycling spent lithium-ion batteries: Revealing and solving deep screening problem
Jiadong Yu, Quanyin Tan, Jinhui Li
Journal of Cleaner Production.2020; 255: 120269. CrossRef
- [Korean]
- Fabrication of TiC powder by carburization of TiH2 powder
-
Hun-Seok Lee, Hyang-Im Seo, Young-Seon Lee, Dong-Jun Lee, Jei-Pil Wang, Dong-Won Lee
-
J Korean Powder Metall Inst. 2017;24(1):29-33. Published online February 1, 2017
-
DOI: https://doi.org/10.4150/KPMI.2017.24.1.29
-
-
684
View
-
2
Download
-
1
Citations
-
 Abstract Abstract
 PDF PDF
Titanium carbide (TiC) powders are successfully synthesized by carburization of titanium hydride (TiH2) powders. The TiH2 powders with size lower than 45 μm (-325 Mesh) are optimally produced by the hydrogenation process, and are mixed with graphite powder by ball milling. The mixtures are then heat-treated in an Ar atmosphere at 800-1200oC for carburization to occur. It has been experimentally and thermodynamically determined that the dehydrogenation, “TiH2 = Ti + H2”, and carburization, “Ti + C = TiC”, occur simultaneously over the reaction temperature range. The unreacted graphite content (free carbon) in each product is precisely measured by acid dissolution and by the filtering method, and it is possible to conclude that the maximal carbon stoichiometry of TiC0.94 is accomplished at 1200°C. -
Citations
Citations to this article as recorded by  - Pre-treatments of initial materials for controlling synthesized TaC characteristics in the SHS process
Jae Jin Sim, Sang Hoon Choi, Ji Hwan Park, Il Kyu Park, Jae Hong Lim, Kyoung Tae Park
journal of Korean Powder Metallurgy Institute.2018; 25(3): 251. CrossRef
|