Search
- Page Path
- HOME > Search
- [Korean]
- Preparation of Porous W-Cu by Freeze Casting of Tert-butyl Alcohol Slurry Mixed with WO3-CuO Powder
- Youngmin Kim, Ji Young Kim, Minju Son, Wonyong Kwon, Eui Seon Lee, Sung-Tag Oh
- J Powder Mater. 2025;32(6):466-471. Published online December 31, 2025
- DOI: https://doi.org/10.4150/jpm.2025.00437
- 614 View
- 6 Download
-
 Abstract
Abstract
 PDF
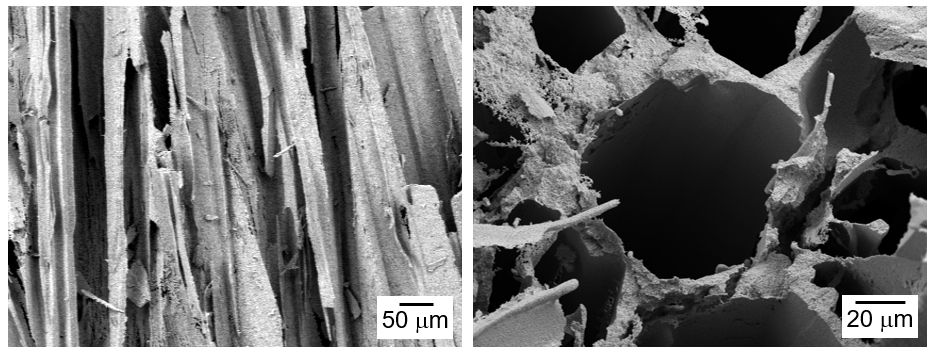
PDF - The influence of process conditions on the microstructure of porous W-Cu, fabricated by freeze casting using tert-butyl alcohol as the freezing agent, was investigated. The slurries containing 10 vol% of WO3-CuO powder were prepared by milling with a small amount of citric acid and polyethylene glycol as dispersants. The slurries with dispersion stability were frozen in a mold with the lower part cooled to -25°C, followed by sublimation in a vacuum to remove the freezing agent. The sintered W-1 vol% Cu in a hydrogen atmosphere exhibited aligned pores with the size of 50 μm, which were generated by sublimation of directionally solidified tert-butyl alcohol crystals. In the cross-section of the specimen, hexagonal pores corresponding to the crystal structure of tert-butyl alcohol was observed. Microstructure analysis of the struts revealed that Cu was distributed non-uniformly due to the mutual insolubility and low wettability of the W-Cu system.
- [Korean]
- Fabrication of 3D Aligned h-BN based Polymer Composites with Enhanced Mechanical Properties for Battery Housing
- Kiho Song, Hyunseung Song, Sang In Lee, Changui Ahn
- J Powder Mater. 2024;31(4):329-335. Published online August 30, 2024
- DOI: https://doi.org/10.4150/jpm.2024.00220
- 1,347 View
- 32 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
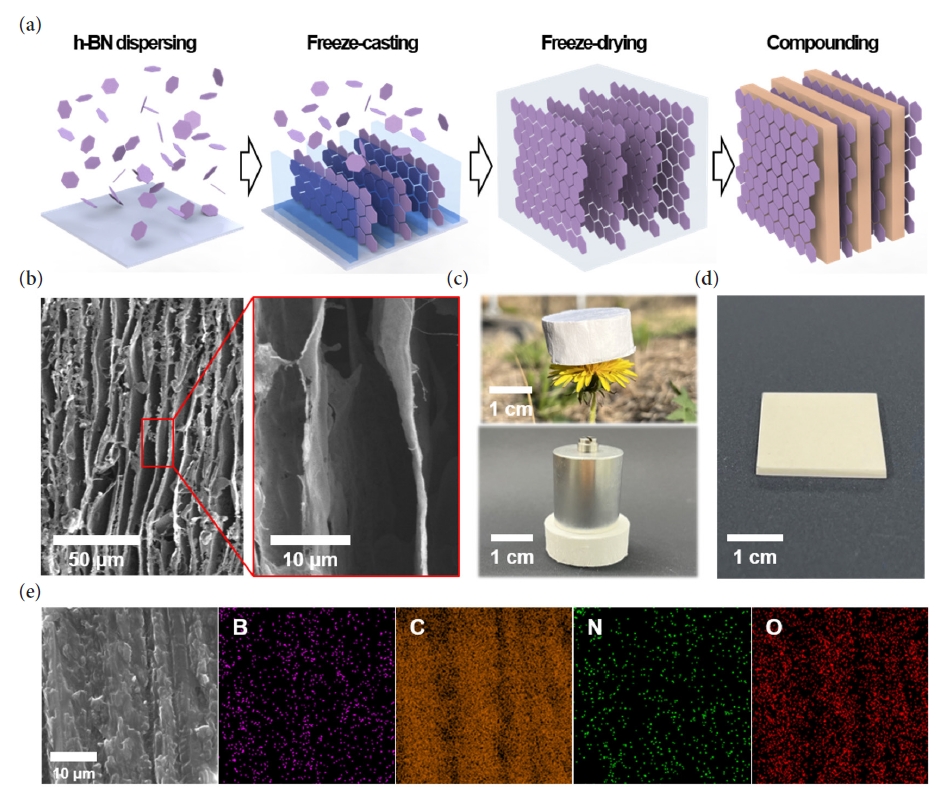
Supplementary Material - As the demand for electric vehicles increases, the stability of batteries has become one of the most significant issues. The battery housing, which protects the battery from external stimuli such as vibration, shock, and heat, is the crucial element in resolving safety problems. Conventional metal battery housings are being converted into polymer composites due to their lightweight and improved corrosion resistance to moisture. The transition to polymer composites requires high mechanical strength, electrical insulation, and thermal stability. In this paper, we proposes a high-strength nanocomposite made by infiltrating epoxy into a 3D aligned h-BN structure. The developed 3D aligned h-BN/epoxy composite not only exhibits a high compressive strength (108 MPa) but also demonstrates excellent electrical insulation and thermal stability, with a stable electrical resistivity at 200 °C and a low thermal expansion coefficient (11.46ⅹppm/℃), respectively.
- [Korean]
- Fabrication of Porous Tungsten by Freeze Casting and Vacuum Drying of WO3/Tert-butyl Alcohol Slurry
- Youn Ji Heo, Eui Seon Lee, Sung-Tag Oh, Young-Keun Jeong
- J Powder Mater. 2022;29(2):118-122. Published online April 1, 2022
- DOI: https://doi.org/10.4150/KPMI.2022.29.2.118

- 895 View
- 11 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF The synthesis of porous W by freeze-casting and vacuum drying is investigated. Ball-milled WO3 powders and tert-butyl alcohol were used as the starting materials. The tert-butyl alcohol slurry is frozen at –25°C and dried under vacuum at –25 and –10°C. The dried bodies are hydrogen-reduced at 800°C and sintered at 1000°C. The XRD analysis shows that WO3 is completely reduced to W without any reaction phases. SEM observations reveal that the struts and pores aligned in the tert-butyl alcohol growth direction, and the change in the powder content and drying temperature affects the pore structure. Furthermore, the struts of the porous body fabricated under vacuum are thinner than those fabricated under atmospheric pressure. This behavior is explained by the growth mechanism of tert-butyl alcohol and rearrangement of the powders during solidification. These results suggest that the pore structure of a porous body can be controlled by the powder content, drying temperature, and pressure.
-
Citations
Citations to this article as recorded by-
Fabrication of porous W by freeze-casting and hydrogen reduction of camphene-based WO
3
suspension
Ji Won Choi, Youngmin Kim, Ji Young Kim, Eui Seon Lee, Sung-Tag Oh
Powder Metallurgy.2025; 68(3): 283. CrossRef - Preparation of Porous W-Cu by Freeze Casting of Tert-butyl Alcohol Slurry Mixed with WO3-CuO Powder
Youngmin Kim, Ji Young Kim, Minju Son, Wonyong Kwon, Eui Seon Lee, Sung-Tag Oh
Journal of Powder Materials.2025; 32(6): 466. CrossRef - Fabrication of Porous TiO2 with Aligned Pores Using Tert-Butyl Alcohol Based Freeze Casting
Eui Seon Lee, Sung-Tag Oh
Korean Journal of Metals and Materials.2024; 62(12): 929. CrossRef
-
Fabrication of porous W by freeze-casting and hydrogen reduction of camphene-based WO
3
suspension
- [Korean]
- Fabrication of Macro-porous Carbon Foams from Spherical Phenolic Resin Powder and Furfuryl Alcohol by Casting Molding
- Hyeondeok Jeong, Seiki Kim
- J Korean Powder Metall Inst. 2019;26(6):502-507. Published online December 1, 2019
- DOI: https://doi.org/10.4150/KPMI.2019.26.6.502
- 525 View
- 1 Download
-
 Abstract
Abstract
 PDF
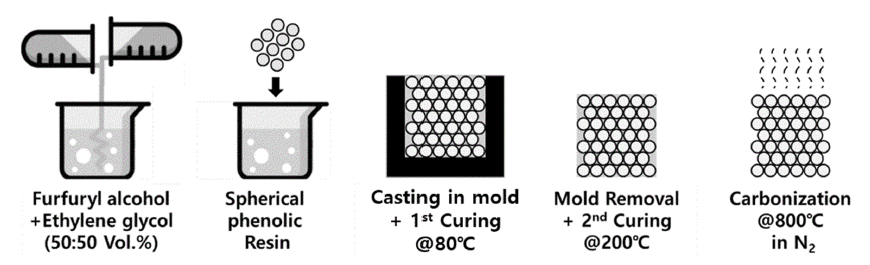
PDF Macro-porous carbon foams are fabricated using cured spherical phenolic resin particles as a matrix and furfuryl alcohol as a binder through a simple casting molding. Different sizes of the phenolic resin particles from 100–450 μm are used to control the pore size and structure. Ethylene glycol is additionally added as a pore-forming agent and oxalic acid is used as an initiator for polymerization of furfuryl alcohol. The polymerization is performed in two steps; at 80°C and 200°C in an ambient atmosphere. The carbonization of the cured body is performed under Nitrogen gas flow (0.8 L/min) at 800°C for 1 h. Shrinkage rate and residual carbon content are measured by size and weight change after carbonization. The pore structures are observed by both electron and optical microscope and compared with the porosity results achieved by the Archimedes method. The porosity is similar regardless of the size of the phenolic resin particles. On the other hand, the pore size increases in proportion to the phenol resin size, which indicates that the pore structure can be controlled by changing the raw material particle size.
- [English]
- Fabrication and Characterization of Thermal Battery using Porous MgO Separator Infiltrated with Li based Molten Salts
- Kyungho Kim, Sungmin Lee, Chae-Nam Im, Seung-Ho Kang, Hae-Won Cheong, Yoonsoo Han
- J Korean Powder Metall Inst. 2017;24(5):364-369. Published online October 1, 2017
- DOI: https://doi.org/10.4150/KPMI.2017.24.5.364
- 803 View
- 9 Download
-
 Abstract
Abstract
 PDF
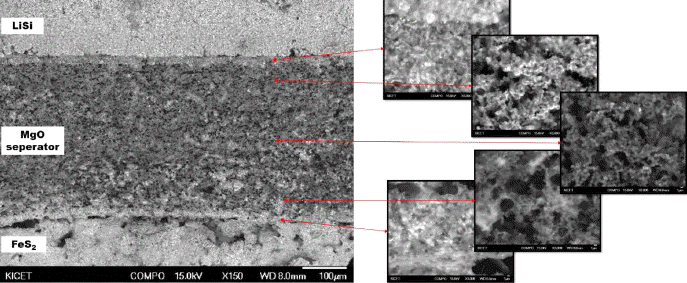
PDF Ceramic powder, such as MgO, is added as a binder to prepare the green compacts of molten salts of an electrolyte for a thermal battery. Despite the addition of a binder, when the thickness of the electrolyte decreases to improve the battery performance, the problem with the unintentional short circuit between the anode and cathode still remains. To improve the current powder molding method, a new type of electrolyte separator with porous MgO preforms is prepared and characteristics of the thermal battery are evaluated. A Spherical PMMA polymer powder is added as a pore-forming agent in the MgO powder, and an organic binder is used to prepare slurry appropriate for tape casting. A porous MgO preform with 300 μm thickness is prepared through a binder burnout and sintering process. The particle size of the starting MgO powder has an effect, not on the porosity of the porous MgO preform, but on the battery characteristics. The porosity of the porous MgO preforms is controlled from 60 to 75% using a pore-forming agent. The batteries prepared using various porosities of preforms show a performance equal to or higher than that of the pellet-shaped battery prepared by the conventional powder molding method.
- [Korean]
- Fabrication of Solid State Electrolyte Li7La3Zr2O12 thick Film by Tape Casting
- Ran-Hee Shin, Samick Son, Sung-Soo Ryu, Hyung-Tae Kim, Yoon-Soo Han
- J Korean Powder Metall Inst. 2016;23(5):379-383. Published online October 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.5.379
- 1,019 View
- 11 Download
- 3 Citations
-
 Abstract
Abstract
 PDF
PDF A thick film of Li7La3Zr2O12 (LLZO) solid-state electrolyte is fabricated using the tape casting process and is compared to a bulk specimen in terms of the density, microstructure, and ion conductivity. The final thickness of LLZO film after sintering is 240 μm which is stacked up with four sheets of LLZO green films including polymeric binders. The relative density of the LLZO film is 83%, which is almost the same as that of the bulk specimen. The ion conductivity of a LLZO thick film is 2.81 × 10−4 S/cm, which is also similar to that of the bulk specimen, 2.54 × 10−4 S/ cm. However, the microstructure shows a large difference in the grain size between the thick film and the bulk specimen. Although the grain boundary area is different between the thick film and the bulk specimen, the fact that both the ion conductivities are very similar means that no secondary phase exists at the grain boundary, which is thought to originate from nonstoichiometry or contamination.
-
Citations
Citations to this article as recorded by- Waste minimization in all-solid-state battery production via re-lithiation of the garnet solid electrolyte LLZO
Vivien Kiyek, Martin Hilger, Melanie Rosen, Jürgen Peter Gross, Markus Mann, Dina Fattakhova-Rohlfing, Ruth Schwaiger, Martin Finsterbusch, Olivier Guillon
Journal of Power Sources.2024; 609: 234709. CrossRef - Powder Aerosol Deposition as a Method to Produce Garnet‐Type Solid Ceramic Electrolytes: A Study on Electrochemical Film Properties and Industrial Applications
Tobias Nazarenus, Yanyan Sun, Jörg Exner, Jaroslaw Kita, Ralf Moos
Energy Technology.2021;[Epub] CrossRef - Synthesize of Nd2Fe14B Powders from 1-D Nd2Fe14B Wires using Electrospinning Process
Nu Si A Eom, Su Noh, Muhammad Aneeq Haq, Bum Sung Kim
Journal of Korean Powder Metallurgy Institute.2019; 26(6): 477. CrossRef
- Waste minimization in all-solid-state battery production via re-lithiation of the garnet solid electrolyte LLZO
- [Korean]
- Briquetting of Waste Silicon Carbide Obtained from Silicon Wafer Sludges
- Seong Mo Koo, Su Jong Yoon, Hye Sung Kim
- J Korean Powder Metall Inst. 2016;23(1):43-48. Published online February 1, 2016
- DOI: https://doi.org/10.4150/KPMI.2016.23.1.43
- 742 View
- 6 Download
-
 Abstract
Abstract
 PDF
PDF Waste SiC powders obtained from silicon wafer sludge have very low density and a narrow particle size distribution of 10-20 μm. A scarce yield of C and Si is expected when SiC powders are incorporated into the Fe melt without briquetting. Here, the briquetting variables of the SiC powders are studied as a function of the sintering temperature, pressure, and type and contents of the binders to improve the yield. It is experimentally confirmed that Si and C from the sintered briquette can be incorporated effectively into the Fe melt when the waste SiC powders milled for 30 min with 20 wt.% Fe binder are sintered at 1100°C upon compaction using a pressure of 250 MPa. XRF-WDS analysis shows that an yield of about 90% is obtained when the SiC briquette is kept in the Fe melt at 1650°C for more than 1 h.
- [Korean]
- Fabrication of Porous Al2O3 Film by Freeze Tape Casting
- Ran-Hee Shin, Jun-Mo Koo, Young-Do Kim, Yoon-Soo Han
- J Korean Powder Metall Inst. 2015;22(6):438-442. Published online December 1, 2015
- DOI: https://doi.org/10.4150/KPMI.2015.22.6.438
- 625 View
- 6 Download
-
 Abstract
Abstract
 PDF
PDF Porous thick film of alumina which is fabricated by freeze tape casting using a camphene-camphor-acrylate vehicle. Alumina slurry is mixed above the melting point of the camphene-camphor solvent. Upon cooling, the camphene-camphor crystallizes from the solution as particle-free dendrites, with the Al2O3 powder and acrylate liquid in the interdendritic spaces. Subsequently, the acrylate liquid is solidified by photopolymerization to offer mechanical properties for handling. The microstructure of the porous alumina film is characterized for systems with different cooling rate around the melting temperature of camphor-camphene. The structure of the dendritic porosity is compared as a function of ratio of camphene-camphor solvent and acrylate content, and Al2O3 powder volume fraction in acrylate in terms of the dendrite arm width.
TOP
 KPMI
KPMI


 First
First Prev
Prev


